Medullary thyroid cancer (MTC) is a neuroendocrine tumor that arises from the calcitonin (CT)-producing thyroid parafollicular C cells, accounting for 3–4% of all thyroid cancers [1, 2]. CT constitutes the most specific and sensitive serum biomarker of MTC and correlates with tumor size [3], whereas different markers markers, such as carcinoembryonic antigen (CEA) and chromogranin A (CgA), are specific but often co-secreted by the tumor [2, 3]. Compared with other thyroid malignancies, MTC exhibits distinctive histopathological features, and the most important immunohistochemical markers are CT and CEA; in fact, in the absence of CT expression, the diagnosis of MTC should be questioned [4]. Also, amyloid material is often present at the histological examination [4]. Calcitonin-negative medullary thyroid cancer (CNMTC), determined by normal serum levels of CT, is a very rare presentation of MTC and only a few cases have been described in the literature [5]. Moreover, CNMTC is often positive for CT immunostaining [6]. In the current case, we report a CNMTC with negative CT, CEA and amyloid substance at the histopathological examination.
A 44-year-old woman presented with asymptomatic neck swelling that was perceived 3 months before. She was receiving atenolol 50 mg once daily to treat high blood pressure and had no personal or family history of thyroid disease or cancer. She did not report any toxic habit. Physical examination revealed an approximately 30 × 20 mm nodule in the left thyroid lobule that moved upwards with swallowing and presented tender consistency. Thyroid stimulating hormone, peripheral thyroid hormones and thyroid peroxidase antibody levels were normal. Ultrasound (US) examination of the neck showed a 30 × 12 × 17 mm well-defined homogeneous hypoechoic solid nodule on the left thyroid lobule with peripheral and central vascularization. No pathological lymph nodes were identified.
According to these findings, fine-needle aspiration (FNA) cytology was performed, showing the presence of a significant number of single follicular cells coexisting with 3D groups of overlapping follicular cells (follicular neoplasm or suspicious for a follicular neoplasm, Bethesda category IV). Consequently, the patient underwent a diagnostic left hemithyroidectomy. Histopathological features were consistent with the presence of a MTC (Figures 1, 2). Despite negative staining for CT, CEA and amyloid substance, epithelial tumor markers (cytokeratins) and neuroendocrine markers (CgA, neuron-specific enolase and synaptophysin) demonstrated strong diffuse staining, whereas the thyroglobulin expression result was negative (Table I).
Table I
Histopathological characteristics of the reported calcitonin-negative medullary thyroid cancer
Figure 1
Medullary thyroid cancer, hematoxylin and eosin staining. A – At low magnification, normal thyroid tissue along with solid, non-encapsulated, ill-defined cell proliferation with infiltrative borders is observed. B – Tumor cell proliferation infiltrating normal thyroid follicles (4×). C – At a higher magnification, tumor cells show spindle morphology and round/oval nuclei with irregular clumps of chromatin
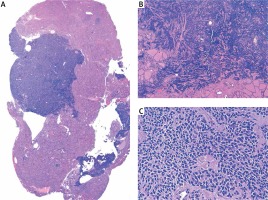
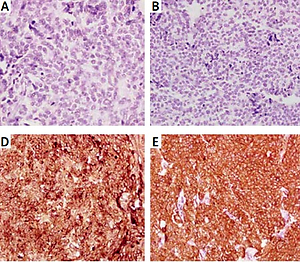
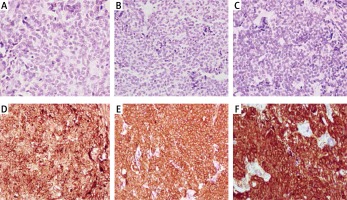
Figure 2
Immunohistochemistry showing tumor cell absent staining for thyroglobulin (A), calcitonin (B) and carcinoembryonic antigen (C) and positivity for chromogranin A (D), synaptophysin (E), and neuron-specific enolase (F)
Additional tests were performed after these results. Whole-body computed tomography did not show any alteration, while somatostatin receptor scintigraphy with 111In-DTPA-octreotide (Octreoscan) only revealed physiological captation in the right thyroid lobule. In the blood test, CT levels were undetectable (< 2 pg/ml, reference range < 10 pg/ml), while CEA (0.22 ng/ml, reference range < 5 ng/ml), CgA (6.2 ng/ml, reference range < 100 ng/ml) and 5-hydroxyindoleacetic acid (3.56 mg/day, reference range < 10 mg/day) levels were within the normal limits. The serum level of thyroglobulin was 12 ng/ml (reference range: 3.5–77 ng/ml). RET genetic screening was negative.
Two months after left hemithyroidectomy, completion thyroidectomy was undertaken. Histopathological evaluation of the right thyroid lobe did not reveal any alteration. Follow-up CT and CEA have remained undetectable and within normal levels, respectively, while US of the neck have not shown signs of local recurrence recurrence after surgery (13 years of follow-up). Periodic computed tomography of the thorax and abdomen did not show evidence of disease.
MTC is an uncommon form of thyroid cancer derived from C cells that is mainly characterized by secretion of CT, a biomarker that constitutes the cornerstone of MTC diagnosis and follow-up [2]. CNMTC is an extremely rare entity and only a few cases have been previously reported. The first case of CNMTC was described by Sobol et al. [7], who identified a thyroid tumor with low levels of serum CT, focal amyloid-like deposits and CT-negative, CgA-positive, CEA-positive immunostaining.
A recent systematic review documented 19 articles including a total of 49 patients with pathological diagnosis of MTC without raised serum levels of CT [6]. Among them, 21 of the 38 cases tested for CT at immunohistochemistry (IHQ) were found to be positive, while CgA-positive staining was found in all the examined cases. Therefore, it is important to note that despite low or normal levels of CT, a significant number of CNMTC presented positivity for CT at IHQ, a fact that has prompted several hypotheses about the pathogenesis of CNMTC, from CT assay interferences to impaired CT secretion by the tumor [8, 9]. In our case, serum levels of CT were not assessed before surgery, since the diagnosis of a MTC was not suspected by the FNA cytopathology. In this sense, discordant FNA interpretation and surgical findings of MTC have been reported in the literature, especially when samples combine preserved cellular cohesion and lack of classic nuclear features of MTC [10]. Importantly, our case presented CT-negative staining in IHQ, which suggests that not only could serum CT secretion mechanisms be altered, but also an impairment in CT synthesis by tumoral cells may be present [11]. In addition, amyloid deposits and CEA were not expressed by the tumor in the current case; consequently, immunohistochemical markers of neuroendocrine differentiation (CgA, neuron-specific enolase and synaptophysin) allowed the diagnosis of MTC. Diffuse or focal positivity for CEA has been reported in almost half of the cases in the literature, while most of them were tumors with amyloid deposits [6]. In fact, in the largest series of CNMTC, nearly 60% of patients presented amyloid deposition in the histopathological evaluation, in spite of the fact that some of them tested negative for CT in IHQ [12]. Amyloid deposition is a distinctive feature of MTC and CT is the only component of this substance [13]; thus alternately processed prohormone of CT or aberrant CT may play a role in the aforementioned cases.
Some authors have argued that the absence of CT secretion implies poor differentiation of the tumor, which may be a sign of adverse prognosis, especially among tumors with CT-negative IHQ [14], although recent studies have shown a very variable prognosis [6]. Our case expressed a low Ki-67 proliferation index, and there was no evidence of regional or distant disease postoperatively; therefore, central lymph node dissection was not performed along with the completion thyroidectomy [4]. In addition, the patient has remained free of disease to date. As regards follow-up, there is no widespread agreement over the most appropriate surveillance strategy. Several authors suggest monitoring serum levels of CT and CEA [15], while neck US, computed tomography and magnetic resonance are the recommended options to detect structural recurrence. Conversely, studies differ about the utility of fluorodeoxyglucose/fluorine 18-dihydroxyphenialanine PET/computed tomography [4, 9].
In conclusion, we report atypical histopathological features in a very unusual form of MTC. These findings reveal that the absence of traditional markers, such as CT, CEA and amyloid deposits, at the pathological evaluation does not rule out the diagnosis of MTC. Also, these characteristics do not seem to determine a worse prognosis.