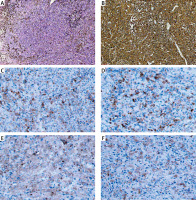
A 69-year-old female patient was hospitalised for irregular vaginal bleeding for over 2 months and painful urination for over 1 month. An ultrasound examination in September 2019 revealed a vaginal mass. The vaginal wall biopsy pathology report dated 16 September 2019 (pathology No.: K1910569) showed malignant melanoma (MM). Immunohistochemistry results were as follows: S-100 (+), HMB45 (+), MelanA (+), CK (−), Ki-67 (20–30%, +), SMA (−), CD4 (−), CD8 (−), CD68 (+) and PD-1 (−) (Figure 1). The patient was clinically diagnosed with stage III vaginal melanoma but refused to undergo genetic testing and anti-tumour treatment for personal reasons. She then underwent two cycles of paclitaxel combined with camrelizumab on 22 October and 9 November 2019. However, she was allergic to paclitaxel; thus, she received two cycles of camrelizumab alone on 26 November and 10 December 2019. In January 2020, the lesion was evaluated as a stable disease (SD) according to the RECIST 1.1 criteria, but the patient did not pursue further treatment for personal reasons.
Figure 1
Pathological diagnosis: malignant melanoma of the vaginal wall. A – Haematoxylin–eosin staining; B – Beta-hydroxy-beta-methylbutyrate staining; C, D – CD4 and CD8 staining: tumour cells are negative, with scattered positive lymphocytes. E – CD68 staining: partially positive. F – PD-1 staining: negative tumour cells and interstitial cells. Micrographs A and B are prepared under 100× magnification while C–F are prepared under 200× magnification
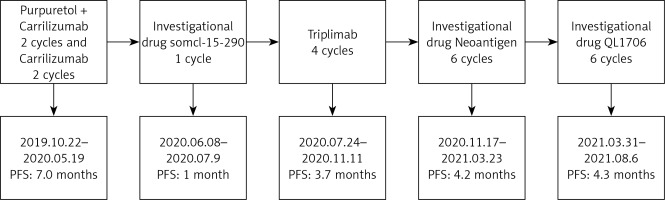
In May 2020, the vaginal tumour had increased in size, with newly detected multiple lymph node metastases in the right inguinal region, indicating tumour progression. On 18 May 2020, the patient visited the gynaecological oncology department and was diagnosed with stage IV MM of the vagina according to International Federation of Gynecology and Obstetrics (FIGO) staging. The patient refused other treatments and opted to participate in a clinical trial of a small molecule inhibitor targeting FGFR/KDR/CSF1R (clinical trial registration number: CTR20191283). After 1 month of treatment with the trial drug SOMCL-15-290, the tumour progressed according to RECIST 1.1 criteria (target lesions increased, and new lesions appeared).
Subsequently, the patient requested to participate in the clinical study entitled ‘Clinical Research on Individualised Neoantigen Tumour Peptide Therapy Based on Genomic Instability Solid Tumours’ (clinical trial registration No.: ChiCTR1900025364). On 20 July 2020, after signing the informed consent, the patient underwent whole exome and transcriptome sequencing of the tumour tissue obtained intraoperatively, as well as whole exome sequencing of the patient’s blood sample. Thus, comprehensive genomic information, including the mutation spectrum of all genes, human leukocyte antigen genotyping and mutated gene expression levels, was obtained. This information was utilised to design effective, specific and safe neoantigen tumour peptides.
While waiting for the screening phase of the neoantigen clinical trial, the patient visited the hospital complaining of defecation difficulties and perineal pain. From 24 July 2020 to 7 October 2020, the patient underwent treatment with trastuzumab injection (240 mg, every 3 weeks) for four cycles. On 11 November 2020, tumour progression was observed according to the RECIST 1.1 criteria (progression-free survival (PFS): 3.7 months). After the tumour progression was found to have no BRFV600 or CKIT mutations, genetic testing was conducted. The tumour mutation burden was 10.437, and the tumour neoantigen burden was 1.472. The patient then received subcutaneous injections of neoantigens (specific types unknown) for six cycles from 17 November 2020 to 2 February 2021. A re-examination on 3 March 2021 revealed an SD, but the target lesion had enlarged compared with the baseline. Accordingly, the patient’s defecation difficulties and local pain worsened. However, the patient and her family requested to withdraw from the clinical trial. On 23 March 2021, the tumour was evaluated as progressing according to the RECIST 1.1 criteria (PFS: 4.2 months).
Eventually, the patient participated in a clinical trial (clinical registration No.: CXSL1900118) involving the combination of anti-PD-1 and anti-CTLA4 monoclonal antibodies engineered to be expressed in a fixed ratio. From 31 March 2021 to 28 July 2021, the patient received the investigational drug QL1706 at 5 mg/kg i.v. ggt every 3 weeks. After two cycles (17 May 2021), tumour assessment according to the RECIST 1.1 criteria showed an SD, with an overall reduction of 23% in the target lesions. The patient experienced significant relief in defecation difficulties and perineal pain. However, on 6 August 2021, the target lesions increased, and a new lymph node metastasis appeared in the right supraclavicular area (PFS: 4.3 months) (Figures 2 and 3). After discontinuing the trial, the patient underwent radiotherapy for the pelvic lesion. During the course of the therapy, the patient experienced perineal pain and low appetite; hence, the treatment was temporarily suspended. Supportive care was provided, and the patient was discharged. However, no further anti-tumour treatment was administered. The patient died on 8 November 2021 as a result of vaginal melanoma progression (overall survival: 21.6 months).
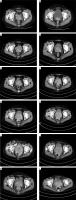
Figure 2
Computed tomographic images of the cervix at different time points during treatment. A–L – October 12, 2019, January 9, 2020, May 19, 2020, July 9, 2020, September 14, 2020, November 11, 2020, January 5, 2021, February 2, 2021, March 2, 2021, March 23, 2021, and May 2021 17 and CT images of the cervical site on June 28, 2021. A – CT image of the cervical site before initial treatment; F – CT image of cervical site before neoantigen treatment; G–I – CT image of cervical site during neoantigen treatment; J – CT image of the cervical site after neoantigen treatment; K, L – CT image of cervical site during investigational drug QL1706 treatment
Although the overall incidence of MM is low in China, its mortality rate is high, and the incidence rate is increasing yearly. The characteristics of MM in Chinese patients differ from those in European Caucasians. In Asians and other people of colour, primary melanoma of the limbs accounts for approximately 50% of MM cases, and mucosal melanoma accounts for 20–30%. Conversely, Caucasian skin melanoma accounts for approximately 90%, with mucosal melanoma tumours representing only 1–5% [1]. Currently, mucosal melanoma is not associated with sun exposure, chemical carcinogens or viral pathogenesis. It commonly occurs in sites such as the genital tract, oral cavity, nasal cavity and conjunctiva and is less common on other mucous membranes [2]. Clinically, mucosal melanoma is often diagnosed already at advanced stages because of its insidious onset, and treatment options are limited, resulting in poor outcomes and low survival rates. Primary melanomas of the female genital tract are rare, accounting for only 1% of all melanomas [3]. Vaginal melanoma has a generally poorer prognosis than cutaneous melanoma and other non-gynaecological mucosal melanomas. Its 5-year survival rate is only approximately 27% [4], whereas that for cutaneous melanoma is roughly 80% [5].
Enhancing treatment outcomes and providing better treatment options for patients with vaginal melanoma is an area that necessitates further exploration. Currently, a gap exists in the clinical understanding of mucosal melanoma, leading to misdiagnosis and inconsistent treatment approaches for some patients. In the current case, the patient did not undergo surgery or treatment recommended by guidelines. However, in clinical practice, patients’ situations are often complex. They may make treatment choices according to factors such as financial constraints, family circumstances and personal preferences. Consequently, patients may not always adhere to the treatment options outlined in the guidelines. In recent years, the number of clinical trials in China has notably increased [6]. With more patients opting to participate in clinical trials, they can have access to a wider range of treatment options. This increase places greater demands on physicians, who are tasked with making informed clinical decisions while considering each patient’s unique circumstances. At the same time, they must guide their patients in making optimal treatment choices.
Mucosal melanoma tends to invade the vascular system [7]. Chemotherapy combined with anti-angiogenic agents remains the recommended treatment approach for non-BRAF V600-mutated mucosal melanoma without surgical indications. However, the PFS time for first-line treatment is only 4 months, and that for second-line treatment is only 2 months [8]. In the present case, the patient received five immunotherapy-related drugs (SOMCL-15-290 has immunomodulatory effects), with four of them demonstrating therapeutic benefits. This case provides several important insights. Firstly, immunotherapy plays a crucial role in melanoma treatment [9]. However, when one of the immunotherapies is ineffective, the way of choosing the next treatment option is unknown. Is it possible to select alternative immunotherapy approaches? Secondly, in cases where multiple lines of immunotherapy are available, how should we sequence and prioritise them in the treatment plan?
Our patient received cancer neoantigen-based anti-tumour therapy starting on 17 November 2020 until tumour progression on 23 March 2021, with a PFS of 4.2 months. This novel antigen therapy appears to have benefited the patient. Research findings suggest that administering anti-PD-1/anti-PD-L1 therapy to early-stage melanoma patients hinders the long-term development of immune responses and impacts tumour immune editing. Considering the sequence of PD-1/PD-L1 therapy in relation to the significance of neoantigens, an alternative approach to rejuvenate exhausted immune cells is proposed [10]. Neoantigens alone are insufficient for a successful immune attack against tumour cells. If the cancer–immunity cycle is disrupted at another stage, the quantity of generated neoantigens becomes less relevant, and neoantigen reduction is associated with immune resistance [11]. To determine whether neoantigen use can help overcome resistance to immune checkpoint inhibitors and identify the optimal treatment sequence for better therapeutic outcomes, we should further understand immune resistance mechanisms and provide more evidence from basic and clinical experiments. Of note, human papillomavirus (HPV) infection is closely linked to various cancers [12]. The relationship between HPV infection and the occurrence, progression and treatment response of vaginal melanoma remains unknown. In the present case, HPV-related information was not tested or available during previous treatments and clinical trial processes; thus, the influence of HPV could not be evaluated. Future clinical work and research should focus on exploring this aspect further.