Introduction
Osteoporosis is a bone disorder characterised by bone loss and destruction of the bone microarchitecture. This imbalance between bone resorption and formation results in fragile bones and increased susceptibility to fracture [1, 2]. Osteoporosis occurs worldwide – mostly in postmenopausal women due to the cessation of oestrogen production by the ovaries [3] and therefore the loss of the positive effect of oestrogen on bone formation [4]. Among the cellular pathways involved in the differentiation and growth of osteoblasts are those of mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) [5]. Osteoclast differentiation and thus bone resorption are controlled by nuclear factor-κB (NF-κB) [6]. Anabolics are used for the management of osteoporosis because they enhance osteoblast function and therefore bone formation. However, conventional treatments for the management of osteoporosis have several limitations. Thus, in the last few decades, interest has grown in the management of osteoporosis by alternative medicine approaches.
Amarogentin is a secoiridoid glycoside that has been isolated from Swertia and Gentiana roots [7]. Swertia mileensis (Gentianaceae) and S. chirayita (Roxb. ex Fleming) are used traditionally in the management of hepatitis, liver injury, and diabetes in China [8]. Amarogentin is reported to have anti-cancer, hepatoprotective, antioxidant, anti-infective, immunomodulatory, anti-inflammatory, and anti-diabetic properties [9–11]. The vasculo-metabolic effects of amarogentin were shown to be mediated by tumour necrosis factor-α (TNF-α) and MAPK [12].
Thus, in this study we used a rat model of oestrogen-deficiency-induced osteoporosis and a human osteoblast cell line to explore whether amarogentin has beneficial effects on osteoporosis.
Material and methods
Animal model
Female Sprague Dawley rats (weight: 200–250 g; age: 3 months) were procured from the Beijing Laboratory Animal Research Center, China, and housed under standard conditions (humidity: 60 ±5%; temperature: 24 ±3°C) with a 12 : 12-h light/dark cycle. All animals were acclimatised to the laboratory conditions and had free access to normal standard chow and tap water. The study protocols were approved by the Institutional Animal Care and Use Committee of Xiaogan Central Hospital, China (IACUC/XCH/2019/14).
Chemicals
Amarogentin was purchased from Sigma-Aldrich Ltd. (St. Louis, MO, USA) and the enzyme-linked immunosorbent assay (ELISA) kits from Thermo Fisher Scientific (Waltham, MA, USA). The primary antibodies used in the western blot assays were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Thermo Fisher Scientific.
Experimental procedures
Bilateral ovariectomy was performed in anaesthetised rats as described previously [13]. A 2-cm incision was made along the abdomen of the rats, and the ovaries were exposed using arterial forceps. After bilateral removal of the ovaries, the abdominal fat pad was repositioned, and the wound was closed by suturing. These rats were assigned to the ovariectomised (OVR) or amarogentin-treated (50 and 100 mg/kg, p.o., for 5 weeks) groups. Rats in the control (sham) group underwent the same surgery but their ovaries were not removed.
Determination of bone mineral density (BMD)
Dual energy X-ray absorptiometry was used to measure the BMD of the rats. Prior to scans of the total femur, lumbar vertebrae, and whole body, the rats were anaesthetised with a mixture of Zoletil-Rompun.
Determination of biochemical parameters
Blood was withdrawn from the retro-orbital plexuses of the anaesthetised rats and centrifuged at 2,000 rpm for 10 min to separate the serum. ELISA kits were used according to the manufacturer’s instructions to measure the serum levels of osteocalcin (OC), C-telopeptide of type 1 collagen (CTX), procollagen type I N-terminal propeptide (PINP), bone-specific alkaline phosphatase (BSAP), interleukin-1β (IL-1β), TNF-α, and IL-17. Bone resorption was estimated based on the concentration of tartrate-resistant acid phosphatase 5b (TRACP 5b).
Western blot assay
Protein was extracted from the right tibia via incubation of the bone in lysis buffer. The lysate was centrifuged at 12,000 rpm for 10 min and the supernatant was collected. The protein concentration was determined using a bicinchoninic acid quantification assay. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% polyacrylamide gel was performed to separate the proteins, which were then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with the following primary antibodies in a western blot assay: Akt (1 : 1,000; Santa Cruz Biotechnology), NF-κB p65 (1 : 100; Thermo Fisher Scientific), Nrf-2 (1 : 500; Santa Cruz Biotechnology), MAPK (1 : 100; Thermo Fisher Scientific), ERK (1 : 200; Thermo Fisher Scientific), and β-actin (1 : 2,000; Santa Cruz Biotechnology). Chemiluminescent signals were detected on the washed membrane using laboratory imaging software.
In vitro study
Determination of alkaline phosphatase activity and osteoblast proliferation
The human osteoblast line MG63 was procured from the American Type Culture Collection, and an immunoassay kit based on the incorporation of bromodeoxyuridine into newly synthesised DNA was used to determine the effect of amarogentin. Alkaline phosphatase activity in pre-osteoblastic MC3T3-E1 cells was estimated using an ALP B-test kit according to the manufacturer’s instructions.
Western blot assay
MG63 cells were treated for 2 h with 100 μg/ml amarogentin with and without ERK inhibitor, after which cell proteins were extracted using PRO-PREP protein extraction solution and subjected to SDS-PAGE on a 10% polyacrylamide gel. The separated proteins were transferred to a PVDF membrane and incubated first with anti-p-ERK (1 : 200; Thermo Fisher Scientific) and anti-ERK (1 : 200; Thermo Fisher Scientific) and then with a horseradish-peroxidase-conjugated secondary antibody. BIO1D software (Scientific Software Group, Salt Lake City, UT, USA) was used for data analysis and a chemiluminescent imaging system was used to visualise the signals on the immunoblots.
Statistical analysis
All data are expressed as the means ± standard error of the mean (n = 8). Results were compared among groups using one-way analysis of variance. A post-hoc comparison of means was carried out using Dunnett’s post-hoc test in Prism software (ver. 6.1; GraphPad Software Inc., San Diego, CA, USA). The level of statistical significance was set at α = 0.05.
Results
Effect of amarogentin on BMD
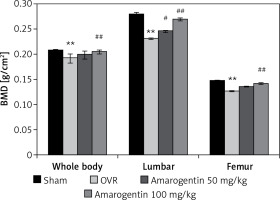
Figure 1 shows the effect of amarogentin on the BMD of the whole body, lumbar spine, and femur of rats with oestrogen-deficiency-induced osteoporosis. The mean BMD of OVR rats was lower than that of sham-operated rats. However, compared to OVR rats, amarogentin-treated rats exhibited enhanced whole-body and lumbar-spine BMD.
Figure 1
Effect of amarogentin on bone mineral density of the whole body, lumbar spine, and femur of rats with oestrogen-deficiency-induced osteoporosis
Data are presented as mean ± standard error of the mean (SEM; n = 8). **p < 0.01, vs. sham control group; #p < 0.05, ##p < 0.01, vs. ovariectomised (OVR) group.
Amarogentin attenuates serum biochemical parameters
The levels of the markers of bone formation in the serum of rats with oestrogen-deficiency-induced osteoporosis that were treated or not treated with amarogentin are shown in Table I. Lower serum OC levels and higher serum CTX, PINP, and BSAP levels were measured in the OVR group than in the sham-operated group. However, amarogentin treatment improved the levels of all four markers in rats with osteoporosis.
Table I
Effect of amarogentin on biochemical markers in the serum of oestrogen-deficiency-induced osteoporosis rat model
| No. | Group | OC [pg/ml] | CTX [ng/ml] | PINP [ng/ml] | BSAP [U/l]) |
|---|---|---|---|---|---|
| 1 | Sham | 8.64 ±0.84 | 39.72 ±2.8 | 6.28 ±0.47 | 9.35 ±0.63 |
| 2 | OVR | 3.49 ±0.27** | 126 ±7.43** | 21.83 ±0.81** | 12.71 ±0.86* |
| 3 | Amarogentin 50 mg/kg | 5.12 ±0.31# | 79.68 ±3.28## | 14.62 ±0.49## | 11.29 ±0.57 |
| 4 | Amarogentin 100 mg/kg | 6.81 ±0.79## | 48.2 ±3.79## | 9.37 ±0.62## | 10.37 ±0.61# |
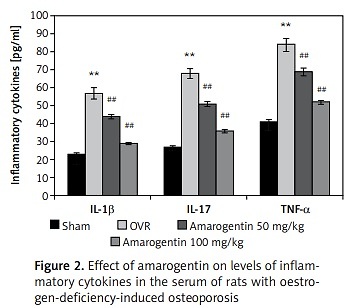
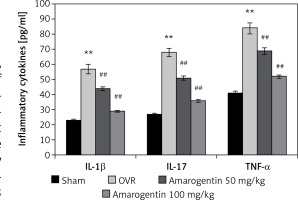
Amarogentin reduces the levels of inflammatory cytokines
The effect of amarogentin on inflammatory cytokine levels in the serum in the rat model is shown in Figure 2. The levels of IL-1β, IL-17, and TNF-α were higher in the OVR group than in the sham-operated group. In the amarogentin-treated group, the levels of all three cytokines were lower than in the OVR group.
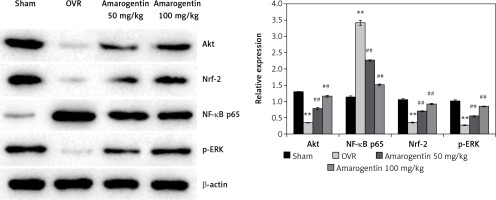
Amarogentin attenuates the expression of Akt, NF-κB p65, Nrf-2, MAPK, and ERK proteins
Figure 3 shows the levels of Akt, NF-κB p65, Nrf-2, and ERK protein expression in the bone tissues of rats with oestrogen-deficiency-induced osteoporosis that were treated with amarogentin. The expression of Akt, Nrf-2, and ERK decreased, whereas that of NF-κB p65 increased in the bone tissues of the OVR rats compared to the sham-operated rats. By contrast, the expression of Akt, Nrf-2, and ERK proteins was significantly enhanced and that of NF-κB p65 reduced in the bone tissues of the amarogentin-treated rats compared to that of the OVR rats.
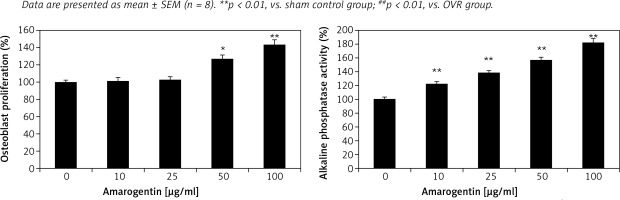
Effect of amarogentin on alkaline phosphatase activity and osteoblast proliferation
Alkaline phosphatase activity and osteoblast proliferation were determined in MG63 cells that were treated or not treated with amarogentin (Figure 4). Amarogentin treatment (50 and 100 μg/ml) significantly enhanced MG63 osteoblast proliferation (p < 0.05 and p < 0.01, respectively) relative to the control group. Alkaline phosphatase activity was also significantly higher (p < 0.01) in amarogentin-treated (10–100 μg/ml) MG63 cells than in the untreated control cells.
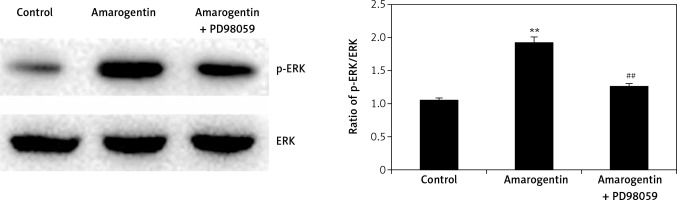
Effect of amarogentin on ERK/MAPK pathway
Figure 5 shows the effect of amarogentin (100 μg/ml) on the ERK/MAPK pathway of MG63 cells in the presence and absence of ERK inhibitor. The ratio of p-ERK/ERK protein expression was significantly higher in the amarogentin-treated group than in the sham-operated group, whereas p-ERK protein expression was significantly reduced in the group treated with amarogentin and an ERK inhibitor.
Discussion
Postmenopausal osteoporosis is the most common bone disorder in females, but current treatments have several limitations [14]. In this study, we evaluated the protective effect of amarogentin both in vivo – against oestrogen-deficiency-induced osteoporosis in rats – and in vitro – in a human osteoblast line. In the rats, serum levels of several biochemical markers of bone formation were evaluated, BMD was measured, and western blot assays of inflammatory cytokines and cellular pathways were conducted. In cultured MG63 cells, the effects of amarogentin on alkaline phosphatase activity and osteoblast proliferation were determined. In addition, the cells were assayed for ERK expression in the presence or absence of an ERK inhibitor.
CTX, OC, BSAP, and PINP are often used as markers of bone formation and resorption [15], and measurements of their levels are used to evaluate drugs designed for the management of osteoporosis [16]. Our study showed that amarogentin treatment attenuates the alterations in CTX, OC, BSAP, and PINP levels in the serum of OVR rats. Inflammatory cytokines play an important role in the regulation of bone resorption, but a deficiency of oestrogen results in their overexpression [17–20]. Our study revealed that amarogentin-treated rats exhibited a significant reduction in the levels of inflammatory cytokines (p < 0.01) compared to OVR rats.
The ERK/MAPK signalling pathway regulates osteoblast apoptosis and differentiation, and ERK participates in osteogenesis and bone metabolism [21]. Physiological inhibition of the ERK gene contributes to the development of osteoporosis by inhibiting osteoblast differentiation and proliferation [22]. Many of the drugs with proven efficacy in the management of osteoporosis modulate the ERK/MAPK signalling pathway [18, 23]. Our study showed that amarogentin treatment enhances osteoblast differentiation in vitro and that ERK expression is reduced in osteoblasts treated with amarogentin and an ERK inhibitor. In addition, the expression of Akt, Nrf-2, and ERK proteins was shown to be significantly enhanced in the bone tissue of OVR rats. Akt and Nrf-2 proteins participate in the development of osteoporosis by altering osteoblast function, as reported previously.
In conclusion, our investigation demonstrated that amarogentin has a protective effect against oestrogen-deficiency-induced osteoporosis in a rat model. Amarogentin was shown to enhance osteoblast differentiation by regulating the Nrf-2/MAPK/ERK signalling pathway.