Introduction
Cardiovascular disorders are the first cause of death in the world, the second is cancer. About 17.9 million people die from cardiovascular diseases (CVDs), an estimated 31% of deaths globally according to data presented by World Health Organization (WHO) in 2017.
CVDs include disorders like coronary heart disease, cerebrovascular disease, rheumatic heart disease, arterial disease, peripheral arterial disease, congenital heart disease, deep vein thrombosis and pulmonary embolism. CVD is the main cause of morbidity and mortality in both men and women in developed countries. Studies have shown that low estrogen levels correlate with coronary artery disease in men [1]. CVD occurs more frequently in men than in women before menopause [2].
As the increased risk of CVD coincides with menopause, studies have shown that female hormones, especially estrogens, are cardioprotective and play significant roles in both reproductive and non-reproductive systems [2]. Menopause is associated with a significant increase of blood pressure, body mass index (BMI), obesity, and body fat distribution [3]. Moreover, they can be synthesized in non-reproductive tissues such as the bone, brain, liver, heart, and muscles [4].
Female sex hormones
Estradiol, which is also known as 17-beta-estradiol or estrogen (E2), is the most common form of circulating estrogens and is also considered to be the primary female sex hormone. Two other forms which are less abundant are estrone (E1) and estriol (E3). The third one (E3) is formed from E1 through 16α-hydroxylation. Their roles become more prominent during pregnancy when they are produced by the placenta in large quantities [5].
Estrogens like other sex hormones are delivered from cholesterol during estrogen biosynthesis [6]. E2 is considered as the major product of this process which plays an important role before menopause, while the significance of E1 grows after menopause [7]. E2 is principally synthesized by ovaries, corpus luteum and placenta but it is also produced by other tissues such as the brain, adipose tissue, bone, vascular endothelium, and aortic smooth muscle cells [5]. E2 synthesized in gonads works to a large extent as a hormonal factor influencing distal tissues, while nongonadal compartments act mainly locally in the tissue in which it is synthesized. Extragonadal production of E2 is crucial because it remains the only source of endogenous E2 in men and postmenopausal women [5].
Dyslipidemia and cholesterol accumulation are more common during menopause, which may be associated with an estrogen deficiency [8]. However, new reports have emerged that show that independent serum estrogen, follicle stimulating hormone (FSH) can increase the production of cholesterol in the liver. A recent study of 278 women found that in perimenopausal women, serum FSH, total cholesterol (TC), and low density lipoprotein cholesterol (LDL-C) levels were higher compared to premenopausal women, despite similar results in serum estrogen levels [9]. Another study involving 588 postmenopausal women confirmed that higher levels of FSH were associated with higher levels in both TC and LDL-C [10]. In a group of 400 postmenopausal women who showed similar values of FSH, TC and LDL-C in the serum, a significant improvement in the level of lipids was noticed only in women after hormone therapy whose FSH concentration decreased by about 30% [11].
Sex steroids have a great effect on the risk of coronary heart disease. Loss of ovarian hormones leads to an increase in LDL-C, triglycerides, and a decrease in high density lipoprotein cholesterol (HDL-C) [12]. These data suggest that blocking FSH signaling lowers serum cholesterol levels by inhibiting hepatic cholesterol biosynthesis [9].
Hormone therapy
The research group comprised women aged 53 to 73 years and not using hormone therapy (HT). Nevertheless, the results of postmenopausal hormone therapy on CVD risk remain controversial [13].
According to the data, oral or transdermal HT does not increase the risk of heart disease. On the contrary, observational studies showed the beneficial cardioprotective effect that can be obtained even with the use of low doses of oral HT (effect of 0.3 mg/day of oral conjugated equine estrogen was similar compared to a standard dose of 0.625 mg/day). The use of HT may delay the progression of the thickness of the intima-media layer of the carotid arteries, which in turn leads to atherosclerosis and coronary calcification [14, 15].
However, the clinical trials confirming the cardioprotective benefits of HT in primary prevention have not been acknowledged yet. In addition, recent data suggest that oral and transdermal HT, in a dose-dependent manner and independent of the HT formulation, may increase the risk of thromboembolism and stroke [13].
For these diseases, transdermal estrogen at a dose < 50 μg/day in combination with micronized progesterone seems to be an innocuous choice. Data suggest that low-dose transdermal and oral HT appears to be safe in relation to CVD risk in menopausal women and during the first years (e.g., 10 years) after menopause. Literature data suggest that TH can alleviate the majority of CVD risk factors to varying degrees, including visceral obesity, dyslipidemia, and glucose hemostasis. Depending on the type of estrogen, dose, route of administration and the type of progestogen, TC, LDL-C, Lp(a) can be lowered and the concentration of HDL-C may be increased [16–18].
When oral estrogen is used, an increase in triglyceride (TG) levels may be observed, however, when administered trans-dermally, TG levels may decrease or remain the same [16, 17]. Furthermore, transdermal estrogen has no effect on the coagulation system and is not associated with an increased risk of venous thromboembolism (VTE) [19].
Non-hormonal drugs are recommended as the first-line therapy for women with an increased baseline thromboembolic risk. If the first-line drugs do not bring the expected result, transdermal estradiol alone or with micronized progesterone application is considered. When HT is initiated > 10 years after the menopause (> 60 years old), because of the risks of coronary heart disease, venous thromboembolism and stroke, HT is recommended to be used for the shortest time conceivable and in the lowest possible dose. Transdermal administration is highly recommended. Nevertheless, individualized pharmacotherapy should be applied when prescribing HT, which includes baseline CVD risk assessment [13].
Most international scientific societies agree that the early initiation of HT in patients with premature or surgical menopause is associated with cardiovascular benefits [20] (Table I).
Table I
Comparison of transdermal E2 and oral CE delivery. Modified [17]
The role of estrogens and estrogen receptors in the cardiovascular system
Growing proof highlights that the synthesis and signaling of estrogens can be both cell- and tissue-specific. It proves that estrogens are not only purely female sex hormones for gonadal organ growth and functioning [7].
Estrogens perform important functions in extragonadal tissues such as the liver, muscles, and the brain. Estrogen treatment is evaluated in clinical trials for dealing with age-related diseases. However, due to large discrepancies in research results, the question arises whether estrogen therapy is beneficial or harmful [7].
Therefore, it is important to understand the exact mechanism of estrogen’s impact on individual tissues in the body. Understanding of cell-specific, tissue estrogen and ERs is essential for studying physiological and pathological changes during aging, as well as being important for the development of new therapeutic agents for the prevention and treatment of heart diseases and other age-related diseases such as Parkinson’s disease (PD), and Alzheimer’s disease (AD) and osteoporosis. Estrogens play an important role in the early development of primary and secondary sexual characteristics. In addition, they are important for the embryonic and fetal development of brain networks. Estrogens have two types of receptors: classical nuclear receptors (ERα and ERβ) and novel cell surface membrane receptors (GPR30 and ER-X) [7].
These two types of estrogen receptors are expressed with tissue- and cell-specific deliveries. Recent trials suggest that brain estrogens are neuroprotective thanks to cell surface membrane receptors and nuclear receptors. Ovarian estrogens play a key role in the management of the reproductive system such as puberty, fertility, and estrous cycle mostly via interaction with nuclear estrogen receptors [7].
During the reproductive period, estrogens secreted by the ovaries have a protective effect on lipid metabolism and the functions of the vascular endothelium. Postmenopausal lower estrogen levels contribute to increased vascular tone through endocrine and autonomic mechanisms that link impaired nitric oxide-dependent vasodilation [21, 22].
The cell- and tissue-specific operation of estrogens as well as their receptors provide healthy aging as well as conflicting outcomes from estrogen therapy in diseases that are related to aging [7].
There is now much evidence of the protective properties of endogenous estrogen against cardiovascular disease (CVD). First, it is known for its positive effect on plasma lipid profiles, as well as antiplatelet and antioxidant properties [23].
Protective role of estrogens
In premenopausal women, estrogens are synthesized mainly in the ovaries, corpus luteum and the placenta. Estrogens are also produced by organs such as the brain, skin, liver, and heart [4]. Three main forms of estrogens are estrone (E1), estradiol (E2, or 17β-estradiol), and estriol (E3) [24].
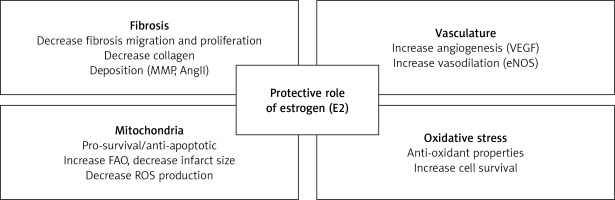
The risk of cardiovascular disease drastically increases after menopause when the level of estrogens is dropping down [25]. The protective role against cardiovascular disease in women during childbearing age is believed to be at least partially related to E2 as endogenous E2 levels and ER expression vary widely between the sexes. E2 is involved in its cardioprotective action by increasing angiogenesis and vasodilation, and by reducing ROS, oxidative stress, and fibrosis [5].
These mechanisms lead E2 to limit heart remodeling and reduce heart hypertrophy. Even if the usage of E2 as a therapeutic agent for human use is controversial, targeting specific ERs in the cardiovascular system could open new and perhaps safer therapeutic options for using E2 to protect the cardiovascular system [5] (Figure 1).
The protective role of estrogen in cardiovascular diseases is associated with a decrease in fibrosis, stimulation of angiogenesis and vasodilation, enhancement of mitochondrial function and reduction in oxidative stress [5].
Studies show that women who experienced premature menopause had a significantly increased risk of a non-fatal cardiovascular event before the age of 60, but not after the age of 70 in comparison to women who reach menopause at the age of 50–51 years. Early menopausal women require close monitoring in clinical practice. Moreover, the age at which a woman reaches menopause may be considered as an important factor in assessing the risk of cardiovascular disease in women. Women who reach menopause at the age under 40 years were categorized as premature menopause, 40–44 years as early menopause, 45–49 years as relatively early, 50–51 years as the reference category, 52–54 years as relatively late and 55 years or older as late menopause. 301 438 women were included in this analysis which was done across five countries and regions (Australia, Scandinavia, the USA, Japan, and the UK) [26].
The menopausal transition is characterized by active changes in follicle-stimulating and estradiol hormone levels. Research done by the Study of Women’s Health Across the Nation (SWAN) and the Melbourne Women’s Midlife Health Project reported a decrease in estradiol level 2 years before the final menstrual period (FMP) and an increase in follicle-stimulating hormone 6 years before [27].
Cardiovascular disease
Women suffer from coronary heart diseases (CHD) a few years later than men. Women are more common to have stable plaques and a better occurrence of microvascular lesions compared to men [28]. Nevertheless, studies have shown that gender is not affecting the outcome of the hypertension treatment [3].
Men get sick more often and are diagnosed with CVD earlier than premenopausal women. After the menopause, the incidence of CVD in women increases significantly [2]. Epidemiological studies have reported that women get sick and die more often from CVD compared to men. There are an increasing number of studies that highlight the gender differences in pharmacokinetics (PK) of drugs for CVD. Differences in drug absorption may come from higher gastric pH in women and the longer gastrointestinal transit time. Differences were also noted in gastrointestinal glutathione S-transferase and cytochrome P450 enzymes. Women have a higher percentage of body fat but weigh less than men, which can have different drug distribution in the body. However, on the topic of pharmacotherapy, there is still a lack of consistent gender findings [29, 30].
The risk increases significantly in the middle age, which interferes with menopause. This observation suggests that the menopause transition (MT) contributes to an increased risk of heart disease [20, 31].
Long-standing studies of menopausal women have provided a better understanding of the relationship between MT and CVD. Research conducted for over 20 years has recognized different patterns of change in endogenous sex hormones and unfavorable changes in body fat distribution, lipids, and lipoproteins, likewise in structural and functional measures of blood vessel health during the MT period [32].
The results highlight the MT period as a colliding time with the CVD risk, which underlines the importance of health monitoring and potential intervention in midlife [20].
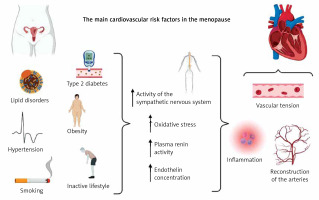
Epidemiological evidence has shown that the most common risk factors for CVD in menopausal women are central obesity, atherogenic dyslipidemia, glucose intolerance and hypertension [33] (Figure 2).
Increased sensitivity to sodium during menopause, which leads to fluid retention in the body causing swelling of the arms and legs, may also contribute to the cardiovascular risk [34].
Hypertension
Hypertension is one of the risk factors for cardiovascular disease [35]. Hypertension affects an increasing proportion of the world population and is one of the leading causes of morbidity and mortality worldwide [36].
Aortic stiffness is considered to lead to death due to cardiovascular events. Degradation of elastin fiber, collagen accumulation, cellular element restructuring and calcium accumulation in the vascular wall, as well as arterial stiffness leads to mechanical adaptation of the arterial wall triggered by hypertensive heart disease [36].
The changes in hormone levels in the vascular system and metabolic changes that occur with age may be directly influenced by the increase in blood pressure, which is more common in postmenopausal women [22]. However, it is not clear whether this is a consequence of the aging process, which reduces vascular flexibility, or menopause [16]. However, several additional factors should be taken into account, i.e., smoking, low physical activity or obesity [37].
Adipose tissue in addition to being an energy storage is also an endocrine organ that produces adipokines such as leptin, resistin, adiponectin, and interleukin 6 [38]. Leptin is one of the biomarkers that may be used as an inflammation factor that may be considered to be responsible for interacting with the arterial wall [36]. Leptin receptors are found in the aorta and blood vessels, and their level is related to the amount of adipose tissue. Studies have shown that that higher levels of leptin in plasma lead to obesity, hypertension, and other heart diseases [36].
Currently available guidelines for the treatment of hypertension should be followed. Nevertheless, the rate of women with high blood pressure is generally under-diagnosed and under-treated [25]. In developed countries, it is estimated that 30% of adult women suffer from hypertension. Younger women have a lower absolute cardiovascular risk compared to older ones. Nonetheless, effective treatment of hypertension should take place at any age. Hypertension occurs twice as often in postmenopausal women as in premenopausal women. Moderate or borderline hypertension (< 140/90 mm Hg) in women affects greater endothelial dysfunction and cardiovascular difficulties compared to men [25].
A recent meta-analysis confirmed that women with early menopause (EM < 45 years) had a higher risk of arterial hypertension (AH) compared to women with menopause > 45 years [39]. This is probably due to a decrease in estrogen levels, which in turn causes the production of vasoconstrictor factors, i.e. endothelin and angiotensinogen, as well as a lower estrogen to androgen ratio during the menopause [40].
Lipid disorders
Women have a higher level of total cholesterol concentration > 6.5 mmol/l compared to 50-year-old males or older in the UK. Raised cholesterol levels are one of the risk factors for heart diseases [25].
The main findings regarding the differences in the lipid profile in women before and after menopause indicate that unfavorable changes in the lipid profile in postmenopausal women expose them to a greater risk of cardiovascular diseases [41].
In the perimenopausal period, changes in the cardiovascular risk usually occur. The occurrence of metabolic syndrome in postmenopausal women is 2–3 times more likely than in premenopausal women [42]. Changes in the lipid profile show a decrease in HDL levels and an increase in the value of triglycerides and LDL cholesterol by about 10–15% in postmenopausal women [43]. An increase in BMI and abdominal obesity in postmenopausal women show about 5 times higher risk of central obesity compared to premenopausal women [44].
Postmenopausal women have increased levels of LDL-C, TC, and TG. Nevertheless, studies indicate that there is no significant difference in HDL-C levels in pre- and postmenopausal women. Future prospective studies are crucial for understanding properly HDL-C levels and functions in a larger group of women with different menopausal status [45].
An independent risk factor for cardiovascular diseases that should be mentioned is Lipoprotein (a) (Lp(a)). Its structure is similar to that of the LDL particle. The differences in structure of these molecules regarding size and electrophoretic mobility are related to the high molecular weight of apo (a). Lp(a) is involved in pro-thrombotic and atherogenic processes. It has the ability to freely penetrate the endothelial barrier and adhere to the arterial wall, which can lead to the development of atherosclerotic cardiovascular disease [46, 47]. Many studies have shown that Lp(a) levels may increase slightly in the perimenopausal period. Although studies are still inconsistent, it cannot be excluded that an elevated Lp(a) concentration may increase the incidence of coronary artery disease in postmenopausal women [48].
An increased incidence of dyslipidemia and cholesterol accumulation can be observed during menopause, traditionally it is associated with estrogen deficit [49]. Due to lack of evidence, the current guidelines for the prevention of heart disease do not contain specific recommendations for men and women separately. Therefore, the latest lipid-lowering guidelines recommend statins as the first-line treatment to reduce the risk of CVD, regardless of gender and menopausal status [47, 50–52].
However, it should be remembered that there are studies suggesting that statin use, especially in high doses, in post-menopausal women may increase the risk of diabates, therefore, the recent recommendations prompt on how to optimally treat lipid disorders in women with metabolic disturbances without incrasing the risk of new onset diabates [53–55]. To reduce the risk of metabolic syndrome, osteoporosis, vascular events and dyslipidemia, lifestyle assessment and counseling for menopausal women should be an integral part of health care. Eating habits and proper care for a healthy lifestyle in the menopausal period are very important because they apply to all women and can be easily modified (Table II).
Table II
Assessment of risk factors for cardiovascular diseases and cardiovascular risk factors at different times of the menopause
| Parameter | Women with surgical menopause at age < 40 y | Women with natural menopause at age < 40 y | Women with menopause at age ≥ 40 y | Menopausal hormone therapy (MHT) |
|---|---|---|---|---|
| Hypertension | ↑↑↑ [32, 52] | ↑↑ [16, 21, 32] | ↑ or ~ [32, 52] | ↓ [32] |
| Coronary heart disease | ↑↑ [32, 52] | ↑ [32, 52] | ↑ [52] | ↓ [32] |
| Heart failure | ↑↑ [52] | ↑ [21, 52] | ~ [32] | ↓ [32] |
| Lipid disorders | ↑↑ [52] | ↑ [21] | ↑ [52] | ↓ or ~ [32] |
| Type 2 diabetes | ↑↑ [17] | ↑ [17, 52] | ↑ or ~ [17, 52] | ↓ [17] |
| Obesity | ↑ [52] | ↑ [21] | ↑ [17] | ↓ [17] |
Postmenopausal women show an increase in blood pressure as well as subclinical vascular disease. This is because of the increased carotid and femoral artery intima media thickness, coronary artery calcium score and arterial stiffness, and impaired flow-mediated dilation. Women with early menopause before the age of 40 have a higher risk of developing diabetes than women with menopause at the age of 50–54 years. Menopause before the age of 40 increases this risk due to more prolonged E2 deficiency. The risk of obesity in women during menopause increases due to a decrease in metabolism and a lack of exercise. These factors can lead to the development of insulin resistance, type 2 diabetes, and many cardiovascular and lipid disorders [17, 20, 56] (Figure 2).
Obesity and diabetes
Diabetes mellitus, despite the advances in early diagnosis, still remains an independent factor showing a very high or high risk of cardiovascular events. This risk is closely correlated with the occurrence of organ damage, e.g. kidney disease. Diabetes is most often diagnosed along with other risk factors for cardiovascular diseases, including dyslipidemia, arterial hypertension or obesity [47]. An increasing number of studies report that the use of menopausal hormonal therapy (MHT) may have a beneficial effect on glucose homeostasis in perimenopausal women with and without type 2 diabetes. A summary of over 100 studies found that MHT reduced abdominal fat in nondiabetic women. In women with diabetes, the use of MHT lowers the level of fasting blood glucose by an average of about 30%, while improving the values of lipids and blood pressure. The beneficial effect of MHT on glucose metabolism is due to the increase in lipid oxidation and energy expenditure. As a consequence, fat storage in the abdominal area is reduced and the risk of central obesity is reduced [57].
In highly developed countries, the problem of obesity and the health burdens it entails are countless. This applies not only to cardiovascular diseases, but also to metabolic and cancerous diseases. Until now, increases in body fat and weight gain have usually been attributed to chronological aging. Current results suggest however that menopause may have an effect on weight and fat gain. During the menopause, there is a decrease in E2 and an increase in FSH. Both of these hormones are responsible for the energy balance, additionally E2 influences the regulation of lipid storage and metabolism in adipose tissue. Therefore, a decrease in E2 during the menopause may contribute to a slower metabolic rate, a decrease in physical activity and the consumption of more kcal. Still, the results of the effects of menopause on obesity are not consistent [58].
Menopause and genetics
Age when natural menopause occurs has a great impact on women’s fertility and health. It is said that the timing of menopause is mostly influenced by genetic factors. Menopausal age ranges usually between 40 and 60 years [59].
Genetic factors play a major role in determining this discrepancy in an age when menopause occurs as has been shown in more than a few mother-daughter studies, twins, and a pair of siblings. Estimated heritability timing of menopause ranges from 31% to 87% and it is a complex genetic trait [59].
In association studies, genes that could be associated with the disease (the so-called candidate genes) are analyzed. This method is based on comparing the frequency of specific alleles and genotype polymorphisms of a given gene in a group of unrelated sick and healthy patients. A given allele may be associated with a disease if it is more common in sick people. Association studies are particularly useful when analyzing multi-gene diseases. Genome-wide association studies show common genetic loci influencing several potential candidate genes in many molecular pathways. A candidate gene’s location is associated with a particular disease. Considering its location, the gene is suspected of causing the disease. These molecular pathways include immune function, ovarian function, neuroendocrine function and DNA repair [20].
Research suggests that genetic predisposition to age at natural menopause (ANM) increases the risk of heart disease in women. Negative genetic correlations between ANM and coronary artery disease and risk factors for cardiovascular disease in women indicate genetic pleiotropy. A few genetic variants related to earlier ANM are linked to increased coronary artery disease and cardiovascular risk factors like hip circumference, and BMI. There were no results that support claims that ANM declining variants have an influence on risk factors for coronary artery disease in men. This demonstrates the usefulness of genetic and gender-specific analyses for a better understanding of the relationship between the cardiovascular risk and ANM [60].
Conclusions
Prevention of cardiovascular diseases should begin early for both men and women. For women, consultations during the perimenopausal and menopausal periods are an ideal opportunity to assess the cardiovascular risk. Early menopause is generally referred to the superior risk of CVD and this factor should be a strong warning sign during a medical consultation [61, 62]. As perimenopause is a time of significant deleterious cardiovascular changes, new research may focus on estimating when a woman will pass the FMP. Anti-Müllerian hormone (AMH) is a new emerging biomarker of reproductive aging which can help to estimate when a woman will undergo her FMP, and, in general, does it better than FSH. Therefore, results of using this new biomarker should complement data gained from other perhaps more traditional hormone measurements [63].