Introduction
Sarcoidosis is a rare, multisystemic, inflammatory disease, characterized by the formation of non-caseating granulomas [1]. Its prevalence differs depending on the geographic location, ethnicity, gender, and age; female gender and age between 25–45 are more likely to be affected [2]. To date, the etiology of sarcoidosis has been unclear but it is speculated that its occurrence is related to both genetic and environmental factors [3].
Being a multisystemic disease, sarcoidosis can affect almost all organs. However pulmonary sarcoidosis shows the highest morbidity rates while at the same time lung is the most frequently affected organ [4]. Except of pulmonary involvement, the cardiac manifestation of the disease is not negligible since its clinical symptoms appear in 3–10% of patients, and its prevalence can reach up to 25% according to autopsy studies [5].
The formation of sarcoid granulomas is attributed to an exaggerated immune response, which begins with the aggregation of immunocompetent cells at sarcoid lesions, the consequent triggering and activation of T cells, and ends with cytokine release [6]. Apart from cell-mediated immunity, there are indications that the humoral branch is also involved in disease development, since pulmonary sarcoidosis is associated with polyclonal hypergammaglobulinemia [7], and a large B cell infiltration in granulomatous tissue of multiple organs suggests their implication in disease regardless of the targeted organ [8].
B-lymphocyte-activating factor (BAFF) belongs to the tumor necrosis factor (TNF) cytokine family [9], and is a type II transmembrane protein expressed by monocytes, macrophages, dendritic cells, bone marrow stroma cells, and T cells as a membrane-bound ligand or as a soluble trimer following cleavage [10]. By binding to the BAFF receptor (BAFF-R), one of its three receptors, signaling cascades are triggered which constitute/makes BAFF a critical factor for the survival and maturation of B cells [11]. BAFF levels in the blood have been found to be elevated in patients with several inflammatory diseases, such as Crohn’s disease (CD) [12], systemic lupus erythematosus (SLE) [13], and multiple sclerosis [14]. In patients with sarcoidosis, serum and bronchial alveolar lavage fluid BAFF levels are increased and positively correlated with the disease’s severity [15, 16] and activity [17]. These findings indicate the possible role of BAFF in the pathogenesis of sarcoidosis, which could be further explored genetically.
Single nucleotide polymorphisms of BAFF and its receptor have been linked to autoimmune diseases, such as SLE [18] and Sjogren’s syndrome [19] in multiple studies, hence confirming their impact on disease development. A BAFF gene polymorphism of interest is rs2893321, an intronic SNP which has been associated with susceptibility to myasthenia gravis [20], Grave’s and autoimmune thyroid diseases [21], and CD [12]. Similarly, two BAFF promoter SNPs, rs1041569 and rs9514828, have been characterized as potential risk factors for chronic lymphocytic leukemia (CLL) [22] and SLE [13, 23], and notably, CD development is strongly correlated with rs1041569 [12]. As for BAFF-R, the missense variant rs61756766 may contribute to increased susceptibility to non-Hodgkin lymphoma [24], CLL [22], Sjogren’s syndrome [25], as well as a higher possibility of developing severe COVID-19 [26]. None of these genetic variants, however, has been investigated for a potential correlation with sarcoidosis susceptibility.
Given the abundance of data linking the aforementioned SNPs with autoimmune and inflammatory disorders, and the lack of their study in the context of sarcoidosis, the aim of this work is to examine the association of BAFF SNPs rs2893321, rs1041569, rs9514828, and BAFF-R SNP rs61756766 with the risk of developing sarcoidosis in a well-defined cohort of Greek patients. Apart from investigating the pulmonary manifestation of the disease, and taking into consideration the high prevalence of cardiac involvement, patients were also grouped as those with and without cardiac involvement. This type of manifestation can be asymptomatic in many cases, thus making it crucial to distinguish potential biomarkers for its presence. This will be the first time that these polymorphisms are studied for their correlation with the disease in a European population, with the purpose of expanding our knowledge on the genetic basis of sarcoidosis.
Material and methods
Sampling
In the present study, 173 patients with sarcoidosis (42 with cardiac involvement and 131 with pulmonary sarcoidosis) of Greek origin were enrolled. The subjects were recruited from one center, the Outpatient Department of Respiratory Medicine, “Attikon” University Hospital, Athens, Greece. Sarcoidosis diagnosis was set based on clinical and radiological criteria and after excluding other possible causes of granulomatosis in transbronchial biopsies with evidence of noncaseating granulomatosis [27]. Of these cases, 21 were newly diagnosed. All patients underwent a complete clinical, detailed cardiac and pulmonary evaluation, which consisted of standard electrocardiography, transthoracic echocardiography, 24 h Holter monitoring, cardiac magnetic resonance imaging, chest X-ray and pulmonary function testing. The modified criteria of the Japanese Ministry of Health and Welfare were used to evaluate the presence of cardiac involvement in sarcoidosis patients [28]. The age- and gender-matched controls were unrelated healthy individuals recruited from Aeginition Hospital, National and Kapodistrian University of Athens, Athens, Greece. The control population consisted of healthy volunteers recruited to attend a health survey representing the Greek population. The study was conducted in accordance with the Declaration of Helsinki, was approved by the Ethical Committee of the participating centers, and subjects signed informed consent to their participation in the study.
Genotyping
Genomic DNA was isolated from peripheral blood samples using the NucleoSpin Blood Kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany), according to the manufacturer’s instructions. In order to genotype the three SNPs of the BAFF gene (rs2893321, rs1041569 and rs9514828), as well as the BAFF-R polymorphism (rs61756766), polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was performed (Kapa Biosystems, Wilmington, MA, USA). Primer sets (Eurofins Genomics AT GmbH, Vienna, Austria) for PCR amplification of BAFF and BAFF-R genes are derived from former studies [21, 29, 30]. All primer sequences used are listed in Table I. The PCR products were digested with restriction enzymes at 37°C overnight and the digestion products were visualized after electrophoresis in 3% agarose gels stained with Gel Red (Biotium, USA). The utilized restriction enzymes and digestion products are listed in Table II.
Table I
Primer sequences and annealing temperatures used for BAFF and BAFF-R SNPs genotyping
Table II
Base changes, digested products and restriction enzymes used for each BAFF and BAFF-R polymorphism
Statistical analysis
All genotypic and haplotype frequencies were determined and compared using SNPStats [31]. Samples were separated into two categories; sarcoidosis patients and control group, and then further divided into sarcoidosis patients with cardiac involvement and without. For each SNP studied, control group samples were tested for departure from Hardy-Weinberg equilibrium. P-values of less than 0.05 were considered to be significant.
Results
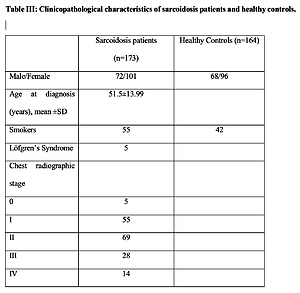
The clinicopathological characteristics and demographics of the study population are listed in Table III. Patients were grouped based on the presence of cardiac sarcoidosis, and notably females had a higher frequency of cardiac involvement in comparison to males.
Table III
Clinicopathological characteristics of sarcoidosis patients and healthy controls
The genotypic frequencies of the studied polymorphisms of the BAFF and BAFF-R genes are listed in Table IV. All frequencies conformed to Hardy-Weinberg equilibrium (p > 0.05). Regarding the rs2893321 polymorphism, there was no significant difference between patients with sarcoidosis and controls. No statistically significant relationship was found for the other two BAFF polymorphisms, rs9514828 and rs1041569; in both cases however, the T allele was overrepresented in sarcoidosis samples (p = 0.03). As for the BAFF-R SNP, rs61756766, the genotype CT and the T allele had a marginally significant association with sarcoidosis (p = 0.05). When stratifying the patients regarding cardiac involvement or not, no association was observed.
Table IV
rs2893321, rs9514828, rs1041569 (BAFF) and rs61756766 (BAFF-R) polymorphism distribution in patients with sarcoidosis
After conducting haplotype analysis using the 3 SNPs of the BAFF gene, 8 haplotypes were formed. Of those, the ATT, GTA and GTT haplotypes frequencies were significantly higher in patients with cardiac involvement compared to patients without (p = 0.012, p = 0.013 and p = 0.024, respectively) (Table V).
Table V
BAFF haplotypes and their frequency in patients with or without cardiac involvement
Discussion
In our study we aimed to discover the potential association of BAFF and BAFF-R SNPs with sarcoidosis. Regarding the polymorphisms of the BAFF gene, none the 3 SNPs studied showed a significant difference of their genotypic frequency between patients and the control group. It is worth noting, however, that the T allele in rs1041569 and rs9514828 appeared more frequently in patients, in both cases. Similarly, the T allele in rs61756766, as well as the CT genotype, were overrepresented in sarcoidosis cases, though with a marginal significance. When patients were stratified based on the presence of cardiac involvement, there was no correlation between the polymorphisms and sarcoidosis or its cardiac manifestation.
Although this is the first time that these polymorphisms were studied for their role in sarcoidosis susceptibility, they have been associated multiple times with several autoimmune disorders. Our findings suggest that rs2893321 is not associated with sarcoidosis, whereas this SNP has been linked in previous studies with Crohn’s disease, where the GG genotype was suggested to have a protective effect [12], myasthenia gravis [20], and Grave’s disease [21], although the last two studies mentioned refer to non-European cohorts. As for rs1041569, we report a significant association between T allele carriers and sarcoidosis. This allele has been detected in higher frequency in cases of myositis (p = 0.029; OR = 1.684, 95% CI: 1.050–2.699) [32] with similar p-value and OR with our results (p = 0.03; OR = 1.43, 95% CI: 1.04–1.98).
Herein, we describe a significant difference in the allelic distribution of rs9514828, and more specifically, an elevated frequency of the T allele in sarcoidosis. Nezos et al. studied rs9514828, among others, and identified this allele as a characteristic of a high-risk group for B-cell lymphoma development in Sjogren’s syndrome [29]. The T allele was also hypothesized to increase susceptibility to SLE in Greek patients [13], yet when it was investigated in a Mexican population, no difference in genotypic or allelic frequency was found [33]. Taken together with our results, these findings suggest a potential role of rs9514828 in immune-related disorders, which differs among ethnic groups.
Regarding the BAFF-R SNP, rs61756766, a marginally significant relationship with sarcoidosis is deduced from our analysis. This mutation has been speculated to cause aberrant signaling through BAFF-R and to contribute to an enhanced activation of NF-κB pathways [24]. Since high BAFF serum levels are a characteristic of active sarcoidosis [17], this variant could be conducive to continuous and defective B cell signaling in this condition. Similar research has strongly linked rs61756766 with Sjogren’s syndrome [25], as well as the CT genotype with a higher risk of CLL [22], which is in agreement with our study. A larger cohort however, could possibly elucidate whether an association actually exists.
The influence of genetic variations in the cardiac manifestation of the disease is not yet known, as limited data are available on the subject. Although the deduction that the particular BAFF and BAFF-R SNPs have an influence on cardiac sarcoidosis cannot be made according to the genotypic distribution presented in this work, haplotype frequency comparison revealed a higher prevalence of 3 BAFF haplotypes (ATT, GTA, GTT) in cardiac sarcoidosis patients. Therefore, BAFF could still have a role in the genetic predisposition of cardiac sarcoidosis development.
In conclusion, this study provides evidence that BAFF and BAFF-R could serve as potential biomarkers for sarcoidosis, independent of cardiac involvement. We believe that this is the first work to link polymorphisms of these genes with sarcoidosis, which could contribute to understanding the genetics of the disease and potentially to developing more efficient treatment strategies. Of course, a larger cohort could be used in a future study to validate our results, and their study in patients of different ethnicity would serve as a further proof of their significance.