Introduction
Low density lipoprotein cholesterol (LDL-C) is a major risk factor for atherosclerotic cardiovascular diseases (ASCVD) [1–4]. Furthermore, a substantial number of clinical studies have demonstrated that lower serum level of LDL-C is associated with better cardiovascular prognosis [5–7]. Besides LDL-C, in the last two decades, some studies have shown that lipoprotein(a) [Lp(a)] might be an independent risk factor for ASCVD [8–10]. However, some studies suggested that after accounting for the effects of LDL-C, the association between Lp(a) and cardiovascular outcome was insignificant [11–14].
Table I
Baseline characteristic comparisons
| Variables | Without MACEs (n = 340) | MACEs (n = 87) |
|---|---|---|
| Male, n (%) | 196 (57.6) | 48 (55.2) |
| Age [years] | 52.2 ±13.8 | 57.9 ±12.2* |
| Current smoker, n (%) | 86 (25.3) | 25 (28.7) |
| Body mass index [kg/m2] | 23.4 ±4.2 | 25.1 ±5.0* |
| Hypertension, n (%) | 144 (42.4) | 37 (44.8) |
| Diabetes mellitus, n (%) | 109 (32.1) | 33 (37.9)* |
| Dyslipidemia, n (%) | 128 (37.6) | 35 (40.2) |
| Atrial fibrillation, n (%) | 16 (4.7) | 6 (6.9) |
| Ischemic stroke, n (%) | 40 (11.8) | 10 (11.5) |
| Peripheral vascular disease, n (%) | 28 (8.2) | 8 (9.2) |
| Chronic heart failure, n (%) | 22 (6.5) | 7 (8.0) |
| Systolic blood pressure [mm Hg] | 129 ±18 | 131 ±20 |
| Diastolic blood pressure [mm Hg] | 70 ±16 | 72 ±19 |
| Heart rate [beats/min] | 78 ±16 | 79 ±14 |
| Total cholesterol [mmol/l] | 4.5 ±0.7 | 4.8 ±0.7 |
| Triglyceride [mmol/l] | 1.6 ±0.6 | 1.7 ±0.8 |
| LDL-C [mmol/l] | 3.0 ±0.8 | 3.5 ±0.6* |
| HDL-C [mmol/l] | 1.1 ±0.4 | 1.0 ±0.3 |
| Lipoprotein(a) [mg/dl]) | 29.8 ±7.6 | 55.6 ±14.3* |
| Creatinine [μmol/l] | 70.4 ±15.2 | 71.9 ±13.8 |
| Glomerular filtration rate [ml/min/1.73 m2] | 81.9 ±10.4 | 78.3 ±9.5 |
| Fasting plasma glucose [mmol/l] | 5.8 ±0.7 | 5.7 ±0.9 |
| Hemoglobin A1c (%) | 6.3 ±0.6 | 6.4 ±0.5 |
| Left main, n (%) | 48 (14.1) | 18 (20.7)* |
| Left anterior descending, n (%) | 117 (34.4) | 32 (36.8) |
| Left circumflex, n (%) | 86 (25.3) | 23 (26.4) |
| Right coronary artery, n (%) | 103 (30.3) | 28 (32.1) |
| Number of stents implanted | 1.5 ±0.7 | 1.6 ±0.8 |
| Drug-eluting stents, n (%) | 335 (95.5) | 82 (94.3) |
| Left ventricular ejection fraction (%) | 52.4 ±8.6 | 50.7 ±7.9 |
Structurally [15], Lp(a) comprises two essential components, namely LDL-C and apolipoprotein(a) [Apo(a)]. Contrary to LDL-C, Lp(a) catabolism is less dependent on LDL-C receptor, and prior studies have shown that statins have a modest effect on Lp(a) regulation [15]. Pathophysiologically [16], compared to LDL-C, Lp(a) is not only capable of promoting atherosclerosis but also capable of enhancing thrombus formation through impairing fibrinolysis. The pathophysiological process of coronary artery stent restenosis is characterized by endothelial dysfunction, smooth muscle cell proliferation and fibrin accumulation [17]. Therefore, with respect to the unique features of Lp(a) in terms of pro-atherosclerosis and pro-thrombosis, one may anticipate that increased serum Lp(a) level could be associated with higher risk of cardiovascular events. However, the data on the association between serum Lp(a) level and cardiovascular outcomes including coronary artery stent restenosis in Chinese populations are limited [18, 19].
Therefore, we conducted a retrospective cohort study to evaluate the association between baseline serum Lp(a) level and cardiovascular outcomes in patients within 1 year after percutaneous coronary intervention (PCI) treatment. We believe that data from our current study will provide insights into the function of Lp(a) as regards cardiovascular outcomes in patients after coronary artery stenting. In addition, our current study also will provide a foundation for clinical trials aimed at reducing cardiovascular events in the future.
Material and methods
Participants’ enrollment
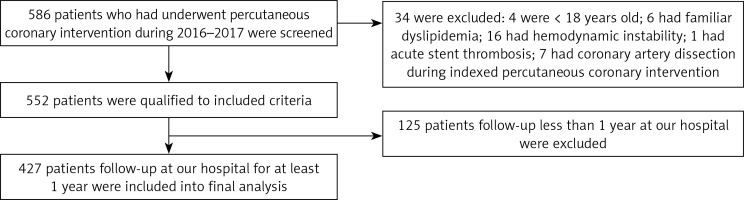
Our current study was approved by the Clinical Research Ethic Committee of Xiang’an Hospital of Xiamen University and the First College of Clinical Medical Sciences of China Three Gorges University. Since this was a retrospective study, no informed consent was required from participants. The inclusion criteria were as follows: ≥ 18 years old, received PCI at our hospital and had follow-up at our hospital within the first year after PCI treatment. The exclusion criteria were as follows: had documented familial dyslipidemia, and had severe complications including hemodynamic instability, acute coronary artery stent thrombosis or coronary artery dissection during indexed PCI. The study schematic diagram is presented in Figure 1.
Data collection
The demographics such as age and gender were collected from the electronic health record (EHR). Body mass index (BMI) was calculated as weight in kilograms divided by height in squared meters. Prior documented medical histories and comorbidities were collected from the EHR. Laboratory parameters were also collected from the EHR. Per hospital protocol, fasting blood samples were drawn before the indexed PCI. Serum level of Lp(a) was measured with a sandwich enzyme linked immune-sorbent assays (ELISA kit, Yaji Biosystems, Shanghai, China). All the procedures were performed in accordance with the manual instructions and were assessed by a SYNCHRON LX20 UniCel DxC800 analyzer (Beckman Coulter Inc., USA). Baseline serum creatinine level was used to calculate the glomerular filtration rate (GFR) in accordance with the Modification of Diet in Renal Disease (MDRD) formula [20].
Studied end points
All participants were followed up for 1 year after the indexed PCI and parameters of interest were collected from the EHR. In specific, studied end points of the current study were the composite of major adverse cardiovascular events (MACEs) including all-cause mortality, non-fatal myocardial infarction (MI), non-fatal ischemic stroke, transient ischemic attack and stent restenosis. All the events were adjudicated by independent cardiologists. All data extraction was conducted by two investigators.
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD) or median (interquartile range, IQR) as appropriate, and were compared by Student’s t-test when data were normally distributed, and otherwise were compared by the Wilcoxon rank-sum test. Categorical data were presented as number (proportion) and compared by the χ2 test or Fisher’s exact test as appropriate. Univariate and multivariate regression analysis was performed to evaluate the association between serum Lp(a) level and MACEs. A Kaplan-Meier survival curve was plotted to evaluate the accumulative incidence of MACEs between normal and increased serum Lp(a) level groups, and the cutoff value was 30 mg/dl in accordance with prior reports [11, 15]. All reported p-values were 2-sided, and a p-value of < 0.05 was considered statistically significant. All statistical analyses were conducted with the SPSS statistical package for Windows version 19.0 (SPSS Inc., Chicago, Illinois).
Results
Participants’ enrollment
Participants’ enrollment was performed from January of 2016 to January of 2017. As presented in Figure 1, a total of 586 patients who underwent PCI in our hospital during this period were screened and 34 were excluded in accordance with the exclusion criteria. Among the 552 patients, 427 who were followed up at our hospital for at least 1 year after the indexed PCI were recruited into the final analysis.
MACEs during 1-year follow-up
During the first year follow-up, a total of 87 (20.4%) MACEs occurred. In specific, 3 (3.4%) patients died, 19 (21.8%) patients had non-fatal MI, 22 (26.3%) patients had non-fatal ischemic stroke, 9 (10.3%) had transient ischemic attack and 34 (39.1%) had stent restenosis. All the events were adjudicated by independent cardiologists. Per study protocol, all patients were divided into two groups, namely without and with MACEs groups, and between-group differences were evaluated.
Baseline characteristics
As presented in Table I, compared to patients who did not have MACEs, patients who had MACEs during the follow-up were older (57.9 ±12.2 years vs. 52.2 ±13.8 years), and were more likely to have higher BMI (25.1 ±5.0 kg/m2 vs. 23.4 ±4.2 kg/m2), diabetes mellitus (37.9% vs. 32.1%) and left main lesion (20.7% vs. 14.1%), and also had higher baseline LDL-C (3.5 ±0.6 mmol/l vs. 3.0 ±0.8 mmol/l) and Lp(a) (55.6 ±14.3 mg/dl vs. 29.8 ±7.6 mg/dl) levels (p < 0.05 for all comparisons). No other significant differences were observed.
Medications used at discharge
As presented in Table II, all patients in both groups were prescribed aspirin and clopidogrel at discharge. Nearly 97.4% and 95.4% of patients in both groups were treated with statins and a higher proportion of patients in the MACE group were treated with ezetimibe than the group without MACE (11.5% vs. 3.5%, p < 0.05). No significant differences in use of other medications were observed.
Table II
Medication use at discharge
| Medications | Without MACEs (n = 340) | MACEs (n = 87) |
|---|---|---|
| Aspirin, n (%) | 340 (100) | 87 (100) |
| Clopidogrel, n (%) | 340 (100) | 87 (100) |
| Statins, n (%) | 331 (97.4) | 83 (95.4) |
| Ezetimibe, n (%) | 12 (3.5) | 10 (11.5)* |
| ACEi/ARB, n (%) | 302 (88.8) | 76 (87.4) |
| β-Blocker, n (%) | 286 (84.1) | 73 (83.9) |
| Oral anti-coagulation, n (%) | 4 (1.2) | 2 (2.3) |
| Calcium channel blocker, n (%) | 83 (24.4) | 20 (23.0) |
| Diuretic, n (%) | 20 (5.9) | 5 (5.7) |
| Oral anti-diabetic medication, n (%) | 65 (19.1) | 18 (20.7) |
| Insulin, n (%) | 33 (9.7) | 10 (11.5) |
Independent risk factors for MACEs
Univariate and multivariate regression analysis were performed to evaluate the independent risk factors for MACEs. As presented in Table III, in the univariate regression analysis, age, current smoking, BMI, diabetes mellitus, LDL-C, Lp(a), GFR and statin use were all independently associated with MACEs. In the multivariate regression analysis, only diabetes mellitus (hazard ratio (HR) = 1.12 and 95% confidence interval (CI): 1.04–1.42), LDL-C (HR = 1.08 and 95% CI: 1.04–1.32), Lp(a) (HR = 1.05 and 95% CI: 1.02–1.24) and GFR (HR = 1.22 and 95% CI: 1.12–1.67) were independent risk factors for MACEs, while statin use (HR = 0.97 and 95% CI: 0.92–0.99) appeared to be a protective factor for MACEs.
Table III
Independent risk factors for MACEs
Accumulative incidence of MACEs by different Lp(a) level
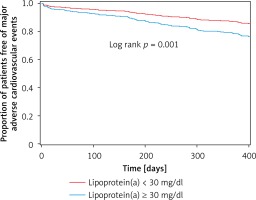
Based on prior reports [10, 15], serum level of Lp(a) < 30 mg/dl was considered as the normal range and accumulative incidence of MACEs was compared between normal and increased Lp(a) level groups. As presented in Figure 2, patients with an increased Lp(a) level had significantly higher incidence of MACEs during the first year’s follow-up than that in the normal Lp(a) level group (p = 0.001).
Discussion
Dyslipidemia, characterized by increased serum LDL-C level, is a major risk factor for ASCVD [21]. Prior randomized clinical trials have demonstrated that a reduced LDL-C level with statins is beneficial to prevent cardiovascular events. However, some studies have shown that despite LDL-C level being within the target range, some patients still have recurrent cardiovascular events. It is speculated that other cholesterol components such as Lp(a) may be associated with residual cardiovascular risk [22]. However, prior findings are conflicting and the data on the Chinese populations are limited. Our current study shows that in the Chinese patients after PCI treatment, independent of LDL-C, baseline serum Lp(a) level is useful to predict 1-year cardiovascular outcomes and increased serum Lp(a) level portends a higher MACE rate than among those with a normal Lp(a) level.
Of note, a substantial number of patients after PCI treatment still experience recurrent cardiovascular events despite serum LDL-C level being within the target range. The underlying mechanisms are multifactorial. Prior studies have shown that increased Lp(a) level might contribute to the residual cardiovascular risk. Indeed, experimental studies suggested that Lp(a) induced endothelial dysfunction, inflammatory reaction and oxidative stress, which in turn led to atherosclerotic initiation and development. Furthermore, some clinical studies, but not all, have also shown that increased Lp(a) level was associated with higher cardiovascular risk. Consistent with prior reports, our current study also showed that among patients after PCI treatment, those who had MACEs during follow-up had higher baseline serum LDL-C and Lp(a) levels. After adjusting for potential confounding factors including LDL-C, Lp(a) remained independently associated with MACEs, indicating that Lp(a) was useful to predict MACEs in the Chinese patients after PCI treatment. However, since our study was a retrospective study, a causal relationship could not be confirmed and future studies are warranted to corroborate our current findings.
Interestingly and importantly, our current study showed that the percentage of stent restenosis was highest compared to other cardiovascular events including all-cause mortality, non-fatal MI, non-fatal ischemic stroke and transient ischemic attack. Since all participants were followed up at our hospital and all participants were compliant with dual antiplatelet therapy during the first year of follow-up after PCI treatment, we considered that the higher stent restenosis risk might not be attributable to noncompliance with dual antiplatelet treatment. In contrast, it might be associated with higher serum Lp(a) level. Indeed, authors [23, 24] reported that in Chinese patients after PCI treatment, baseline increased LDL-C and Lp(a) portended a higher risk of revascularization. Consistent with their study, our current study also found that those with increased Lp(a) level had higher stent restenosis risk. However, in the prior study, after adjusting for baseline LDL-C level, the association between Lp(a) and risk of coronary revascularization was insignificant. In our current study, we found that even after adjusting for potential covariates including LDL-C, increased serum Lp(a) level at baseline remained significantly associated with stent restenosis, with a hazard ratio of 1.16 (95% CI: 1.04–1.25, p = 0.013). Unfortunately, although our study and other studies have shown the association between Lp(a) and cardiovascular events including stent restenosis, no effective and efficient approach could be used to reduce Lp(a) level up till now.
With respect to our preliminary findings, future directions should be focused on corroborating our findings from the national level of China as well as in other population groups. Basic research should be conducted to evaluate the underlying mechanisms. In addition, future randomized, prospective, large-scale trials are necessary to demonstrate whether reducing the Lp(a) level can prevent cardiovascular events in patients with an achieved LDL-C target after treatment [23, 24].
Some limitations of our current study should be mentioned: first of all, this was a retrospective study which was subjected to the limitation of temporal relationship in terms of the association between exposure and outcomes; second, the observational design could not allow us to infer a causal relationship; third, although we adjusted for potential confounding factors, unmeasured and undetected confounding factors still existed which could have impacted the results; fourth, our current study concerned a retrospective cohort and no LDL-C was obtained after finishing follow-up. However, our current study was focused on the baseline LDL-C and Lp(a) levels in relation to cardiovascular outcomes. In addition, in the regression analysis model, we adjusted for baseline LDL-C level. In the future prospective cohort study, it is necessary to evaluate LDL-C change during follow-up; last but not the least, our current study was conducted in a Chinese population and therefore future studies performed in other racial/ethnic populations are warranted to corroborate our findings.
In conclusion, our current study shows that in Chinese patients with PCI treatment, baseline serum Lp(a) level can be used to predict MACEs, especially stent restenosis, and all these findings are independent of baseline LDL-C level. Future randomized trials are necessary to demonstrate whether reducing the Lp(a) level can further prevent cardiovascular events in patients with an achieved LDL-C target.