Lyme disease typically occurs in 3 stages: early localized disease, early disseminated disease with organ involvement, and late disseminated disease [1]. Lyme neuroborreliosis (LNB) can occur in both early and late disseminated phases. A confirmed diagnosis can be made when all three conditions are met: (1) the patient has neurological symptoms typical of neuroborreliosis, (2) pleocytosis in the cerebrospinal fluid (CSF), and (3) development of antibodies against Borrelia burgdorferi in the CSF [2]. The typical neurological symptoms of LNB include headaches and cranial nerve paralysis or paresis, and rarely radiculitis and peripheral neuritis, peripheral polyneuropathy, and chronic encephalomyelitis [3]. The first case of diaphragm paralysis as a complication of Lyme disease was reported in 1986 [4]. In the current literature, 16 cases of neuroborreliosis complicated by paralysis of the diaphragm have been reported, out of which 4 cases concerned bilateral paralysis [5]. Therefore, the presented case will be the fifth described case of bilateral diaphragmatic paralysis in the course of probable Lyme neuroborreliosis.
A 64-year-old male patient, with a history of arterial hypertension, hyperlipidemia, emphysema, and an incident of pulmonary embolism (6 years prior) was admitted to the Department of Internal Medicine with symptoms of acute respiratory failure occurring in the supine position – the patient complained of dyspnea and breathing disorders during sleep, with oxygen saturation (SpO2) decreasing to 75–80% in the supine position.
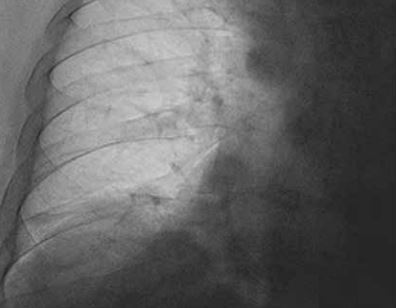
In addition, the patient complained of pain in the cervical spine for about a month. Upon admission, physical examination revealed tachypnea, a thoracic breathing pattern, and increased respiratory effort with the activation of accessory respiratory muscles, without any signs of an acute respiratory infection. Arterial blood gases indicated hypoxemic respiratory failure, the inflammatory markers were low and the D-dimer concentration was normal. Medical imaging was carried out: computed tomography pulmonary angiography (angio-CT) excluded the diagnosis of pulmonary embolism, computed tomography (CT) of the chest showed features of emphysema but excluded the diagnosis of pneumonia, and the CT of the cervical spine and the echocardiography did not reveal any significant abnormalities. An X-ray of the chest showed asymmetry of the diaphragm with the elevation of the left hemidiaphragm. Bronchoscopy was performed, in sitting and supine positions, and no abnormalities or differences between the positions were found. According to the recommendations from the neurological consultation, brain magnetic resonance imaging (MRI) with contrast was performed, showing no significant pathologies. The consulting pulmonologist suspected left-sided phrenic nerve palsy and suggested carrying out a chest fluoroscopy.
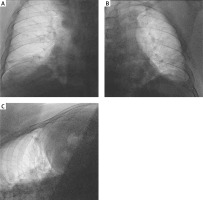
The patient was transferred to the Department of Cardiology of a University Hospital, where chest fluoroscopy was available in the radiodiagnostics. The examination revealed bilateral phrenic nerve palsy (Figure 1). During the hospitalization, the patient required oxygen therapy via a nasal cannula, with a flow of oxygen of approx. 2 l/min during the night. Due to the unclear etiology of the palsy, the patient was transferred to the Department of Neurology of a University Hospital for further diagnostics and treatment.
Figure 1
Chest fluoroscopy showing the bilateral phrenic nerve palsy. A – The right side of the chest cavity (anteroposterior (AP) view, supine position). B – The left side of the chest cavity (AP view, supine position). C – The right side of the chest cavity (AP view, partially rotated supine position)
Upon admission to the Department of Neurology, the patient presented with dyspnea at rest and the SpO2 in the sitting position was 90%. Dyspnea intensified in the supine position, with SpO2 decreasing to 86%. An increase in the respiratory rate and paradoxical respiratory movements of the abdominal cavity were also observed. Neurological examination revealed no significant abnormalities. MRI of the cervical spine was performed showing cervical spondylosis, however not providing any answer about the etiology of the presented symptoms. During differential diagnosis, an electromyography test was performed to exclude myasthenia gravis, the result of which was negative. The patient’s electroneuronography revealed L4, L5, and S1 radiculopathy with no changes in the nerve conduction study of the cervical region.
The diagnostic process was broadened and serological analysis was carried out to test for a number of antibodies: anti-gangliosides, anti-myelin oligodendrocyte glycoprotein (MOG), and anti-aquaporin to exclude autoimmune neuromyelitis, anti-acetylcholine receptor and anti-muscle specific kinase (MuSK) to exclude myasthenia gravis. Tests for antibodies specific to both types of herpes simplex virus antigens (HSV), cytomegalovirus (CMV), VDRL (venereal disease research laboratory) and antibodies specific to Lyme disease were also performed. While the tests for auto-antibodies, HSV, CMV, VDRL were negative, a positive result was obtained for both IgM (> 190 AU/ml) and IgG (145 AU/ml) antibodies in the blood serum, specific of Borrelia spirochete antigens. The patient’s medical history was reevaluated and there was no history of tick bites that the patient was aware of.
Next, a lumbar puncture was performed. The analysis of the basic CSF parameters was normal, however, oligoclonal bands were found in the CSF, and ELISA and Western Blot revealed positive IgM and IgG antibodies specific of B. burgdorferi antigens. The diagnostic criteria for LNB were analyzed – two out of three criteria were required for a confirmed diagnosis (1. presence of neurological symptoms typical of neuroborreliosis, which cannot be explained by a different cause, 2. CSF pleocytosis, 3. production of antibodies specific of B. burgdorferi in the CSF) were met [2]. Hence, the patient was diagnosed with bilateral phrenic nerve palsy in the course of probable Lyme neuroborreliosis.
Targeted antibiotic therapy against B. burgdorferi was introduced. The patient was treated with intravenous ceftriaxone (1 × 2 g/day). During the hospitalization, partial clinical improvement was achieved and the patient was discharged home in a stable condition without any respiratory support. The recommendations upon discharge included continuation of oral antibiotic therapy with cefuroxime (2 × 500 mg/day) for 3 more weeks under the supervision of the Neurological Outpatient Clinic.
Acute respiratory failure, worsening in the supine position, can be caused by diaphragmatic paralysis, which needs to be taken into account as a differential diagnosis. Chest fluoroscopy is a test that allows assessing the motion and function of the diaphragm in real time. In the course of differential diagnosis of the causes of phrenic nerve palsy, we should take into account the patient’s history of cardio-thoracic surgery, compression, and compression lesions, for example in the case of lung and mediastinal tumors. They need to be especially considered in the case of unilateral phrenic nerve palsy. Other possible causes of phrenic nerve palsy include polyneuropathy and critical state polyneuropathy in patients in intensive care. Molecular LNB diagnostics, which involves the detection of spirochete DNA using PCR was not performed. According to the EFNS (European Federation of Neurological Societies) recommendations, a direct method of identification is recommended when the antibody index is negative [6, 7]. Molecular diagnostics has its limitations due to the small number of spirochetes in the CSF and the high affinity of bacteria to the myelin sheath [8]. Additionally, there is no gold standard for detecting LNB using the PCR method and the criteria for LNB diagnosing include serological confirmation. We are aware that unidentified microbes that were not included in the diagnostic process could have contributed to the patient’s atypical symptoms, and the antibiotic used in the patient was a wide-spectrum antibiotic. However, the patient met the criteria for the diagnosis of a probable Lyme neuroborreliosis, the remaining inflammatory parameters were negative, therefore the patient was diagnosed with bilateral phrenic nerve palsy, probably in the course of neuroborreliosis.
The EFNS guidelines recommend that a patient with a definite or probable diagnosis of LNB, both in its early and late forms, should be treated with ceftriaxone i.v., doxycycline p.o. or cefotaxime i.v. Those antibiotics are recommended because of their effectiveness and safety, and the available research shows that all three antibiotics have similar effectiveness [9]. The analysis of cases of neuroborreliosis with paralysis of the phrenic nerves available in the literature, revealed that intravenous ceftriaxone was the most frequent choice. Therefore, we have also chosen this antibiotic to treat the patient described in this case.
In conclusion, palsy of the phrenic nerve in the course of LNB is a rare and unique symptom. What is of great significance is the treatability of the cause. Therefore, it seems reasonable to consider serological diagnostics for Lyme disease in patients with diaphragmatic paralysis of undetermined etiology.