The recommendations of Polish clinical experts (the Polish Headache Society – PHS, the Headache Section of the Polish Neurological Society – HS PNS) regarding migraine treatment, published by Stępień et al. in 2021, in which the opinions of Polish, European, and American opinion-leaders were taken into account, state that a very good safety profile and high clinical efficacy make anti-CGRP-pathway monoclonal antibodies (CGRP/CGRP-R – mAbs) the first-line drugs for the prophylactic treatment of migraine. This includes the prevention of chronic migraine (CM), which makes it now equal to onabotulinumtoxinA (ONA-BoNTA) as far as the decision making point for CM therapy is concerned, and equal to classical oral preventives – for migraine in general [1] (Table I). Such a revolutionary turn in the therapeutic approach to treatment of migraine is based not only on the data of the clinical outcomes of treatment using this group of drugs, but also considering their safety and tolerance issues. In the new recommendations of the HS PNS (recently prepared for publication in Polish) based on the most recent RCT (randomized controlled trial) publications and Polish experts’ opinions and experience, the authors specifically point out the high efficacy and very good safety profile and tolerance of the new CGRP/CGRP-R drugs. These therapeutics can be used not only for many months but even for years.
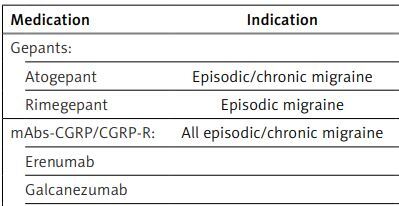
Table I
Drugs used for prophylactic treatment of migraine, indications, doses, and first (A) or next (B) class according PHS/HS PNS recommendation (in order from the newest in Poland)
Existing oral therapies. Failures in classical oral prophylactic therapies of migraine are mainly associated with unacceptable adverse events, which appear in over 40% of patients, contraindications to particular medications, as well as with too short a period of treatment in the real world, inadequate dose, and parallel abuse of analgesics leading to concomitant medication overuse headache (MOH). Consensus says that the minimum period of prophylactic treatment with classical oral preventives should be at least 3 months; however, some drugs and/or some patients may require a longer trial, which may not be possible. The contemporary approach to therapies takes into consideration not only efficacy with the “number needed to treat” (NNT), but also safety and tolerance with the “number needed to harm” (NNH), and such a switch significantly changes the perspectives and paradigms of treatment.
Anti-CGRP/CGRP receptor monoclonal antibodies. The authors elucidate that the use of CGRP/CGRP-R mAbs in the prophylactic treatment of migraine is approved in patients 18 years of age and older, diagnosed with chronic migraine according to the ICHD-3 criteria, or episodic migraine with 4 or more headache days per month (monthly headache days; MHD). There are currently no data on the efficacy and safety of this treatment in children and the elderly. Long-term studies did not reveal drug-related adverse events other than those noted in the registration studies. The European Headache Federation (EHF) recommendations for the treatment of chronic migraine with CGRP/CGRP-R mAbs do not recommend their use in pregnant and breastfeeding women, among individuals with cardiovascular diseases, in those who abuse alcohol and drugs, as well as in patients with mental disorders [2]. The mAbs are directed either against the CGRP receptor (erenumab) or against the CGRP ligand (fremanezumab, galcanezumab, eptinezumab). In all registration studies, very good safety and tolerance, along with high efficacy in reducing headache days and migraine attacks and number of days, have been demonstrated. Due to their high efficacy, good safety, and tolerance, quick onset of action (within a week, on average), no need for titration of the dose, and excellent convenience related to only one injection every month or every 3 months (triple dose of fremanezumab or quarterly infusion of eptinezumab), these therapies are associated with very good compliance and adherence. Even though both the American and European consensus point to such high efficacy of biological therapies, the American Headache Society (AHS) consensus published in 2021 recommended initiation of mAbs against CGRP/CGRP-R as a second-line therapy following inability to tolerate or inadequate response to 2 or more classical oral preventives (topiramate, divalproex sodium/valproate sodium, β-blockers, tricyclic antidepressants, venlafaxine, duloxetine) in episodic migraine, or 2 or more of these drugs or onabotulinumtoxin A in chronic migraine [3]. Moreover, the European consensus statement on the diagnosis and management of migraine positioned both mAbs and ONA-BoNTA into the third-line medications as the classical oral preventives were divided into 2 separate lines of drugs [4]. A significant change in the paradigm of the therapeutic approach was first published with the update of the European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene-related peptide pathway for migraine prevention in 2022, in which the authors suggested mAbs targeting the CGRP pathway to be included as a first-line treatment option [5, 6].
Onabotulinumtoxin A. The aforementioned ONA-BoNTA was shown to be safe, well tolerated, and effective in the prophylactic treatment of chronic migraine with negative recommendation in episodic form. Such a neurotoxin, known to cause chemical denervation of muscles via inhibition of presynaptic release of acetylcholine, and widely used in dystonia and spasticity, seems to act in the prophylaxis of migraine through inhibition of presynaptic release of CGRP and other neurotransmitters with a potential role in the pathogenesis of migraine. The evidence of its efficacy in chronic migraine was demonstrated in the pivotal PREEMPT study, leading to FDA approval soon afterwards; its efficacy, good safety, and excellent tolerance were also confirmed by numerous real-world data (RWD) in subsequent years.
The position of non-oral medication in chronic migraine. The positioning of “newer” non-oral therapeutic options in chronic migraine is controversial because there have been no “head-to-head” clinical trials. In the absence of studies directly comparing ONA-BoNTA and anti-CGRP/CGRP-R–mAbs, conclusions about its efficacy might be drawn from meta-analyses. Unfortunately, 2 of them that have recently been published have methodological flaws. Both included clinical trials for chronic as well as episodic migraine, while it is known that ONA-BoNTA is ineffective in episodic cases. There were also significantly different numbers of patients treated with these 2 methods taken for analysis, which makes these studies incomparable because of different statistical power of particular groups. Even though the total dose per session of ONA-BoNTA in the USA is 155 U, and in Europe 155–195 U, these meta-analyses took into account trials using 50, through 100, up to 255 U, which is not only illegible, but also results in incorrect numbers and locations of the officially approved injection points. Moreover, the trials taken into account had different basic characteristics in terms of allowed/not allowed concomitant prophylactic therapies, as well as abortive drugs from very different groups of medications. In view of the mentioned methodological concerns, comparing and positioning of ONA-BoNTA vs. anti-CGRP pathway mAbs is not justified [7, 8]. Nevertheless, both European and American consensus and recommendations state significant efficacy, safety, and tolerance of ONA-BoNTA and mAbs targeting the CGRP pathway versus placebo, and they quote both therapies as comparable, without positioning either of them as superior to the other [2–6]. However, both therapies are definitely superior to topiramate, which is also effective, but related with significantly more common adverse events leading to frequent discontinuation of the therapy [8]. What is interesting is the trend to apply dual therapy (ONA-BoNTA + mAb against CGRP pathway) in resistant/refractory patients with no response to monotherapy with each of these treatments alone [9].
Gepants. Gepants (second generation) are relatively new drugs, which are antagonists of the CGRP receptor. These drugs are non-peptide small molecules approved as an abortive and/or prophylactic treatment of migraine with or without aura. Currently, oral ubrogepant (for acute therapy), rimegepant (for acute and prophylactic therapy), atogepant (for prophylactic therapy), and intranasal zavegepant (for rescue therapy) are used in the treatment of migraine. Rimegepant was the first gepant registered in Europe, including Poland. At the time of preparing the manuscript of this statement a second gepant was registered in Europe/Poland, which is atogepant. The remaining gepants (along with rimegepant and atogepant) are currently available only in the US.
Rimegepant is indicated for the abortive treatment of episodic and chronic migraine, but also as a preventive medication, but currently only in episodic migraine. Gepants are similarly effective in the emergency treatment of migraine as triptans. They differ from the later by their absence of a negative impact on the cardiovascular system and thus are alternatives to triptans in patients with risk factors for cardiovascular disorders. We recommend gepants for the abortive treatment of migraine attacks in adult patients with a diagnosis of migraine with/without aura or chronic migraine, but specifically in patients with contraindications to triptans, poor tolerance to them, or inadequate response to treatment with 2 or more triptans. Coexistence of frequent migraine attacks and MOH related to triptans is another indication to gepants, especially because these drugs do not lead to the development of MOH independently of the number of the days they are taken per month.
In pivotal studies, all FDA-approved gepants showed similar clinical efficacy and very good safety and tolerance, specifically with no hepatotoxicity reported earlier with the first-generation of these molecules. It is assumed that the lack of cardiovascular contraindications and very good safety and tolerance of gepants are due to their mechanism of action, which is significantly different from that of triptans, which, as selective presynaptic agonists of 5HT1B/1D receptors “spare the vessels” (comparing to nonselective serotonin receptor agonist ergotamine), still constrict the arteries to some extent. That is why they are contraindicated in post-stroke and/or post-myocardial infarction patients, and in persons with ischaemic heart disease or uncontrolled hypertensive disease. Conversely, gepants, which block the CGRP receptors involved in physiological vasodilation as well as the pathogenesis of migraine, do not constrict the arteries but only prevent vasodilation along with inhibition of neurogenic inflammation. As well as the action on these 2 undesired phenomena happening in the course of migraine attacks, gepants also inhibit CGRP release at neuronal synapses leading to inhibition of nociceptive transmission. Thus, gepants can be safely used in patients with previous cardiovascular events, and in people in whom triptans are contraindicated, poorly tolerated, or ineffective. However, animal studies have shown a toxic effect of gepants on the foetus, so they are not registered for pregnant or lactating women. For safety reasons, rimegepant is not recommended for patients with hepatic or renal insufficiency.
Rimegepant taken abortively not only significantly diminishes the intensity of headaches comparing to placebo, but also significantly decreases “the most bothersome symptoms”. The clinical effect is rapid and reaches statistical significance in just 1 h. In patients who used rimegepant frequently it was noticed that the drug not only led to the development of MOH but also decreased the average number of headache days per month and monthly migraine days. This observation underlies subsequent clinical trials with rimegepant for the prevention of migraine, which achieved good primary and secondary outcomes ending up with registration of this compound for prophylactic treatment of episodic migraine. For abortive indications the drug should be taken at a dose 75 mg, and only one dose per 24 h is allowed. For prevention, 75 mg should be taken every other day. In case of a migraine attack on a day between the scheduled dose days, the patient may take an extra pill on that day, but not more than 75 mg per day is allowed. The drug proved to be safe, well tolerated, and continuously efficient [https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212728s000lbl.pdf].
Rimegepant is metabolized by CYP3A4 and by CYP2C9, which must be kept in mind in case other drugs are used concomitantly. It is eliminated unchanged, and excreted in faeces and urine. The half-life is 11 h. Rimegepant is not registered for children and adolescents, but clinical trials (with promising positive results presented at the American Academy of Neurology Congress in 2023) on these cohorts are ongoing [10].
Atogepant is the second gepant registered in Europe. It has been approved for the preventive treatment in both episodic and chronic migraine following 2 pivotal RCTs: the ADVANCE trial (EM)[11] and the PROGRESS trial (CM) [12]. The drug is available in tablets in 2 doses: 60 mg – for routine administration daily, and 10 mg – for patients with concomitant severe renal disfunction, as well as in the case of concomitant use of potent inhibitors of CYP2A4 and/or OATP. In patients with mild or moderate liver disfunction, reduction of the dose is not necessary; however, in severe liver disfunction atogepant should be avoided. There are currently no data on the safety and efficacy of the drug in children and adolescence. The drug is not recommended during pregnancy, and as far as the treatment of breastfeeding women is concerned it should be balanced between foreseen advantages for a migrainous mother and her breastfed child versus potential harm to the child (no data from humans).
In conclusion, as far as strategies of abortive and prophylactic treatment are concerned, following European and American guidelines, we suggest (migraine attack treatment) starting with large doses of simple analgesics and/or NSAIDS in mild intensity migraine attacks, but with triptans or gepants (rimegepant) in moderate and severe attacks. Gepants (rimegepant) should definitely be considered, at least in individuals with contraindications or poor tolerance of triptans or with unsatisfactory effect of the latter. The general rule “the sooner the better” or “treat when mild” is a reasonable approach to treat migraine attacks because it allows a better and quicker effect.
In preventive treatment 2-line therapies can be applied, starting with classical oral preventives (from at least 2 different groups of drugs), and then switching to mAbs targeting the CGRP pathway or gepants (rimegepant, atogepant) – for episodic migraine, or switching to ONA-BoNTA or mAbs against CGRP/CGRP-R or gepant (atogepant) – for chronic migraine, in case of lack of efficacy, poor tolerance, or contraindications. However, taking into account numerous data from RCTs and RWDs, especially considering the safety and tolerance of the therapies as well as the “NNT vs. NNH issue”, as Polish experts we suggest considering a change of the 2-line therapy strategy, because it might be worth initiating migraine prophylaxis with not only efficient but safer and better tolerated therapies. Thus, considering mAbs targeting the CGRP pathway or CGRP receptor antagonists – gepants (rimegepant or atogepant) for episodic migraine, and ONA-BoNTA or anti-CGRP/CGRP-R mAbs or atogepant for chronic migraine as “first-line” therapies, might guarantee much better compliance and adherence to these therapies, as well as long-term satisfaction of patients right from the beginning of the treatment. Even though there might be limitations to this strategy due to higher costs of the “newer” medications, taking into account pharmacoeconomic implications both for particular individuals as well as for the national health system and for society (including absenteeism, presenteeism, high costs of abortive drugs if taken frequently, developing MOH, costs of consultations, hospitalizations, and investigative procedures) as well as quick and constant improvement of quality of live, the new paradigm proposed here may be much more beneficial than the existing “traditional”, 2-line-therapy strategy.
Conflict of interest
ID has served as an expert on Advisory Boards and as a lecturer for the following companies: Allergan/Abbvie, Novartis, Teva, Elli Lily, Pfizer; WK has served as an expert on Advisory Boards and as a lecturer for the following companies: Allerga/Abbvie, Novartis, Teva, Elli Lily Pfizer; AS has served as an expert on Advisory Boards and as a lecturer for the following companies: Allergan/Abbvie, Novartis, Teva, Elli Lily Pfizer; JJR has served as an expert on Advisory Boards and as a lecturer for the following companies: Allergan/Abbvie, Novartis, Teva, Elli Lily, Pfizer; MBJ has contracted advisory boards, consultations, and lectures for Allergan/Abbvie, Pfizer, Novartis, Polpharma, and Teva.