Transient global amnesia (TGA) is a transient disorder of short-term memory with an acute onset that spontaneously resolves within 24 h, often leaving a gap in memory of several hours. The aetiology is unknown. None of the hypotheses attempting to explain the pathophysiological basis of TGA is without flaws. The hypothesis based on cortical spreading depression (CSD) currently appears to be the most credible [1, 2]. TGA occurs most frequently in the 6th to 7th decade of life and is more common in women. The onset of symptoms is often preceded by exposure to severe psychological stress (more often in women) or physical exertion (more often in men). In a head MRI performed 24 h later, some patients exhibit punctate hyperintense lesions in the DWI sequence (absent in the acute phase of the disease) located in one or both hippocampi, which disappear in the following days. The prognosis is good, recurrences occur rarely, and treatment is not needed [2].
High blood pressure values are often recorded in patients during the acute phase of TGA [3]. Disturbances in heart rate variability have also been demonstrated in patients after a TGA episode [4]. These symptoms suggest transient disturbances in autonomic nervous system function with increased sympathetic activation; however, it is unclear whether these abnormalities precede TGA onset or are a consequence of it. Activation of the sympathetic nervous system and the secretion of catecholamines (adrenaline, noradrenaline) caused by stress, along with stimulation of the hypothalamic-pituitary-adrenal axis with increased secretion of ACTH and cortisol, enhance blood coagulability through increased activity of factors V, VIII, and von Willebrand factor. Stress-induced hypercoagulability, unlike hemodynamic changes (blood pressure, heart rate), which quickly resolve after the stressor is removed, takes longer to normalize. Increased physical activity similarly enhances blood coagulability as does acute psychological stress [5–9]. Activated partial thromboplastin time (aPTT) can be shortened by 7–38% following acute physical exertion [6].
The study aimed to evaluate blood coagulation parameters in patients during the acute phase of TGA.
Methods
A retrospective, cross-sectional analysis was conducted on the medical records of 84 patients diagnosed with TGA hospitalized between 2014 and 2024 in a single neurology department in Poland. Inclusion criteria included documented results of aPTT, prothrombin time (PT), fibrinogen and D-dimer levels measured within 24 h from the onset of symptoms (acute phase). Exclusion criteria included a medical history with coagulopathies, an embolic-thrombotic episode in the past 3 months, anticoagulant use, and liver diseases. A total of 62 patients were included for further analysis, including 4 with recurrent TGA. All measurements were performed in the same laboratory, which accepted the following reference ranges: aPTT 28–39 s, PT 11–14 s, D-dimers < 500 ng/ml, fibrinogen 2.0–4.0 g/l.
Results
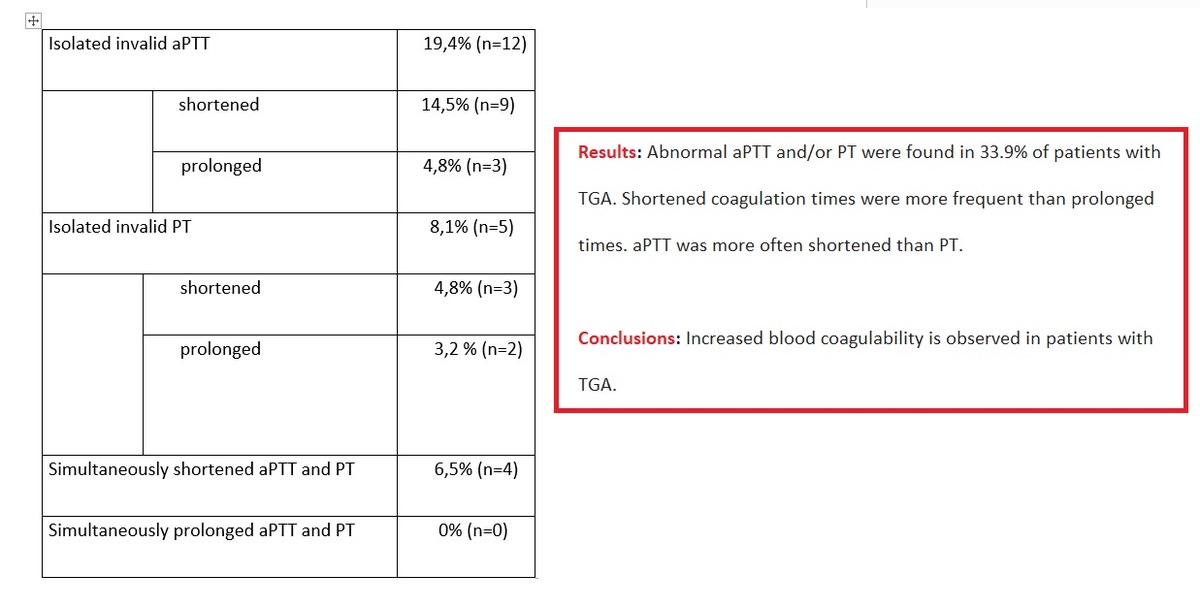
The analysed group showed an overrepresentation of women (women : men = 43:19), with a mean age of 65.8 ±7.9 (40–88) years. In 33.9% (n = 21) of patients (women : men = 15 : 6), abnormal aPTT and/or PT were noted (Table I), with elevated D-dimer levels in 11.2% (n = 7) (maximum 3–4 times) and fibrinogen abnormalities in 3.2% (n = 2). Among patients with abnormal aPTT (n = 16), 81% (n = 13) had a shortened aPPT (26.0 ±1.7 [21.8–27.7] s) and 19% (n = 3) had a prolonged aPPT (55.0 ±12.8 [45.9–64.0] s). PT abnormalities were less frequent (n = 9); 78% (n = 7) had a shortened PT (10.4 ±0.4 [9.8–10.9] s) and 22% (n = 2) had a prolonged PT (14.6–15.8 s). In 75% (n = 3) of patients with recurrent TGA, simultaneous shortening of aPTT and PT was recorded, without deviations in D-dimer and fibrinogen levels. In 14.5% (n = 9) of patients (those < 60 years old or with recurrent TGA), during hospitalization, the levels of protein C, protein S, antithrombin III, and lupus anticoagulant (anti-LA) were measured as part of extended diagnostics and the results were normal. Additionally, genetic testing for the factor V Leiden mutation and the G20210A prothrombin gene mutation was performed in 2 patients with recurrent TGA and no mutations were found.
Table I
Disturbances in clotting times in patients with TGA
Discussion
The study showed that 1/3 of patients with TGA in the acute phase had disrupted blood coagulation times. The authors did not find other studies directly investigating coagulation disorders in TGA patients with which they could compare their results. The findings are pioneering and outline new research directions regarding the pathophysiology of TGA. aPTT serves as an indicator of the functioning of the intrinsic pathway of the coagulation system (factors XII, XI, IX, and VIII) and also depends on factors involved in thrombin formation (II, V, X) and the conversion of fibrinogen to fibrin. PT reflects the efficiency of the extrinsic coagulation system (V, VII, X) and factor II and fibrinogen [10]. Shortened aPTT and/or PT occurs in states of hypercoagulability and may also result from improper blood sample collection or transport, hyperactivity of coagulation factors, while prolonged coagulation times may arise from the use of anticoagulants, the presence of lupus anticoagulant, or deficiencies of coagulation factors (e.g., in liver diseases or vitamin K deficiency) [11, 12]. Fibrinogen is essential for clot formation (as it is also an acute phase protein, its concentration can increase in inflammatory states), while D-dimer levels increase as a result of the fibrinolysis process. Disruptions were more commonly observed in aPTT than in PT. Shortening of aPTT or PT was more frequent than their prolongation. Simultaneous shortening of aPTT and PT was observed in 3/4 of patients with recurrent TGA, suggesting a potential link to the risk of disease recurrence. Elevated levels of D-dimers and fibrinogen were noted sporadically. No thromboembolic or hemorrhagic complications were observed in any of the patients during hospitalization. Extended diagnostics for the causes of increased blood coagulability were only performed in some patients with abnormal coagulation times (a limitation arising from the retrospective nature of the study) and no abnormalities in protein C, protein S, antithrombin III, or anti-LA were found in any of the patients. The transient state of increased blood coagulability, blood pressure peaks, and heart rhythm disturbances (symptoms documented to be associated with the acute phase of TGA) may result from sympathetic nervous system activation, for instance, due to stress or physical exertion – both recognized factors frequently preceding the occurrence of TGA. Shortened aPTT is a risk factor for ischemic stroke and worsens the prognosis regarding functional status [13].
The study results suggest that some patients in the acute phase of TGA exhibit blood clotting abnormalities. The hippocampus, a key structure in the pathogenesis of TGA, is also the most vulnerable brain structure to ischemia and oxidative stress. Increased blood coagulability could potentially contribute to the formation of microthrombi, which may lead to transient dysfunction of the hippocampi (e.g., by triggering CSD). It is possible that individuals with a tendency toward excessive coagulation system activation in response to stressors are more susceptible to TGA, especially when high blood pressure is also present (which adds risk of blood-brain barrier damage). Alternative hypotheses include:
– TGA activates the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis, and the transient hypercoagulable state is a consequence of this activation; or
– abnormal coagulation times are a nonspecific marker resulting from other factors unrelated directly to TGA (e.g., dehydration due to stress).
Further studies are necessary to answer these questions, involving larger groups of patients from diverse ethnic backgrounds [14]. These studies should include comparisons with control groups (to assess whether a similar proportion of shortened coagulation times occurs in healthy individuals or after other paroxysmal episodes, such as TIA or epileptic seizures) and evaluate the time required for coagulation times to normalize.
The main limitations of the study include a relatively small and ethnically homogeneous study group, its retrospective nature, the absence of a control group, and the lack of serial monitoring of blood clotting times in the days following the acute phase of TGA.