Cervical cancer is the great concern and usually develops slowly. Cervical intraepithelial neoplasia (CIN) is a precursor to cervical cancer, and its progression, particularly from CIN2 to invasive carcinoma, is typically slow. The disease typically progresses through a precursor stage known as cervical intraepithelial neoplasia, which is classified into three grades (CIN1-3) based on the extent of dysplasia and its potential for malignant transformation. Persistent infection with high-risk human papillomavirus (HPV), particularly HPV16 and HPV18, is recognized as the primary etiological factor in cervical carcinogenesis.
While CIN1 lesions frequently regress spontaneously, CIN2 and CIN3 have a higher risk of progression to invasive carcinoma, necessitating close monitoring and appropriate management. Early detection through regular screening is essential for effective management and prevention of disease progression. Individual cases of rapid CIN progression challenge the standard screening protocols such as cytological evaluation, highlighting the need for further investigation into potential biomarkers that could complement existing screening protocols. Carcinoembryonic antigen (CEA) is a tumor marker that has been suggested to have a potential role in the progression of cervical neoplasia, although its significance remains unclear.
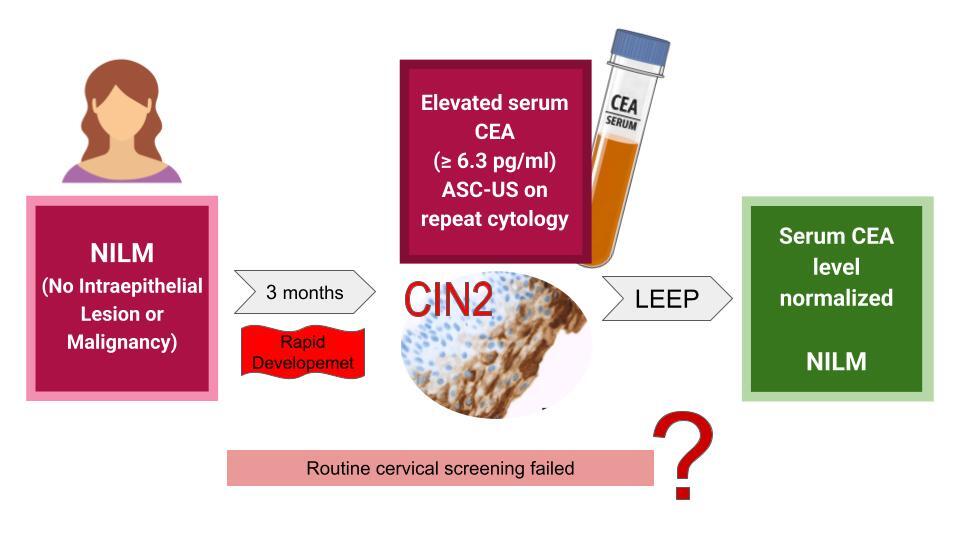
A 32-year-old female patient presented to the ambulatory practice with an elevated serum level of CEA at 6.3 pg/ml (normal reference range < 4.3 pg/ml). Her medical history was unremarkable, including no prior surgeries, a single delivery, and no known gynecological or endocrinological conditions. She was not on any medications, and her previous cytological examinations had all been normal. The CEA test was conducted occasionally as a part of tumor marker monitoring as part of the family’s health insurance package.
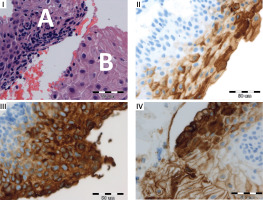
Diagnostic investigations were subsequently extended at an oncological center, where comprehensive examinations, including lungs, gastrointestinal system and thyroid, yielded normal results, including a computed tomography scan. Additionally gynecological consultation was suggested. Three months prior to the examination, the patient had undergone a cervical smear with a result of 1A2A (NILM) in the Bethesda System. A repeated cytology was performed after the CEA elevation, revealing a result of 1A2C3C with ASCUS (Atypical Squamous Cells of Undetermined Significance). Due to the atypical findings, the patient underwent a biopsy of the uterine cervix and curettage of the cervical canal, which confirmed the presence of cervical intraepithelial neoplasia grade 2 (CIN2) (Figure 1, biopsy specimens and CEA expression). During the biopsy, a colposcopy was performed using 3% acetic acid and Lugol’s iodine staining, which revealed a 3 × 5 mm area of epithelial whitening with moderate iodine negativity, consistent with HSIL.
Figure 1
Biopsy specimens and CEA expression. I. Upper left: Hematoxylin and eosin stain showing epithelial dysplasia (A) and non-dysplastic tissue (B). II. Upper right: Normal cervical squamous epithelium with weak to moderate CEA positivity of the superficial layer. III. Lower left: Strong CEA expression in dysplastic epithelium. IV. Lower right: CEA expression was absent to weak (shallowly) in normal tissue. More intense CEA staining in dysplastic tissue. Acknowledgments: Authors would like to thank the pathologist, Konstanty Korski MD PhD (Roche Diagnostics GmbH, Penzberg, Germany and Department of Pathology Greater Poland Cancer Centre, Garbary Street, Poznan, Poland) for additional molecular staining
To mitigate the risk of further dysplastic progression, a loop electrosurgical excision procedure (LEEP) with total pathology removal was performed. Postoperative results showed ectopia glandularis and no further dysplastic lesions were found (entirely evaluated as ICD-O 8077/2). Serum CEA levels normalized a month later in the same laboratory. The patient remained disease-free with normal cytological findings for the subsequent 5 years.
CEA is known to be overexpressed in various malignancies including colon, breast and ovarian cancer. It has been mainly used as a prognostic marker correlated with advanced stages of colorectal cancer. The expression of CEA may serve as a potentially valuable diagnostic tool for assessing the risk of progressive CIN. However, the findings in the literature on this topic remain controversial. An investigation by Kainz et al. on HPV infection and CEA expression in CIN2 involving 32 women revealed that CEA was present in 91% of cervical tissue samples [1]. Similarly, an analysis by Luo et al. of 80 cases of high-grade cervical glandular intraepithelial neoplasia (HCGIN) demonstrated that CEA was expressed in 63.8% of cases [2].
On the other hand, Tendler et al. demonstrated that significant CEA expression in cervical biopsy specimens was observed only in high-grade CIN, with no correlation between increased cellular CEA expression and serum CEA levels [3]. Even in cervical cancer patients with markedly elevated cellular CEA, increased serum CEA levels remain exceedingly rare, occurring in only approximately 0.09% of cases. CEA expression was found to increase between CIN2 and CIN3, however this was not accompanied by elevations in serum CEA. These findings suggest that cellular CEA expression may serve as an indicator of progressive cervical neoplasia. A separate case-control study concluded that CEA expression was not a reliable marker for distinguishing CIN from normal cervical epithelium [4].
Elevated serum CEA levels are uncommon in the early stages of cervical neoplasia, as such cases have not been reported so far. A significant increase typically occurs later, in advanced neoplastic conditions. In contrast, CA19-9 is typically elevated specifically in CIN3, as indicated by another study [5]. These patterns raise the question whether certain biomarkers could help distinguish high-grade CIN from early invasive cancer. Nevertheless, the data in Table I show slightly higher frequencies of autoantibodies against CEA in CIN3 compared to CIN1 and CIN2, suggesting a potential link between CEA expression and neoplastic progression. It is therefore worth considering whether elevated CEA levels could, in some cases, indicate unusually rapid progression of cervical intraepithelial neoplasia.
Table I
Frequencies of autoantibodies against CA15-3, CEA, CA19-9, c-Myc, p53, Hsp27 and Hsp70 TAAs in normal, CIN1, CIN2, CIN3 and cancer groups according to Jin et al. [5]
Another hypothesis was proposed by Becker et al. whose study showed that indirect mechanisms, such as immune dysregulation associated with SARS-CoV-2 infection, could potentially accelerate carcinogenesis. The patient probably developed microinvasive carcinoma from CIN2 within 3 months, being infected 3 months before the follow-up visit and with a recovery time of 4 months but the virus was not detectable in cervical tissue [6]. That is why we decided to treat it aggressively. Similar remarks have also been made by Bedell et al. by mentioning that CIN1 caused by HPV not always proceeds to CIN3, because CIN3 and eventually cancer can also develop in previously normal epithelium, bypassing a low grade dysplasia stage or even simultaneously to CIN1 [7]. This phenomenon is called a “molecular switch” model and could explain such a rapid development of cancer in our patient assuming HPV infection as she had no history of HPV vaccination. Another possible explanation is proposed by Nedjai et al. who found that DNA methylations and series of epigenetic changes taking place both before and throughout the HPV infection are strongly associated with and characteristic of the lesion grade, because they promote amplification and genetic instability of the virus [8]. Therefore, they contribute to the varied morphological outcomes, such as CIN1 or CIN3, helping to explain the differences in the progression of these lesions.While histological staining and p16 overexpression hold prognostic value in stratification of patients as low/high-risk groups to progression/recurrence CIN2 [9], standard screening methods unfortunately failed to detect the rapid advancement in the presented patient.
There is also a reported case of rapid recurrence of differentiated invasive squamous cell carcinoma (SCC) of the uterine cervix in the patient with relapsing remitting multiple sclerosis (RRMS) taking natalizumab [10]. After radical abdominal hysterectomy and no signs of residual CIN or carcinoma, recurrent cervical SCC occurred within 2 years, supposedly due to immunosuppression. The issue of more frequent screening for immunocompromised women was also raised, since WHO and ACOG recommendations of 3-year interval would not be enough for 2-year progression, but still too long for our patient. Therefore, in spite of national screening programs, we should always remain vigilant and be ready for non-standardized management if circumstances arise, for example, of an isolated elevated CEA marker.
Therefore, it is crucial to assess, manage and treat each case individually, sometimes extending beyond standard protocols. While routine cervical screening is undeniably effective, we should always remain alert to atypical cases that may not follow expected patterns. Some patients may present unusual levels of protein expression or altered metabolism due to unknown factors, leading to an elevated marker level, that should not typically occur in a non-cancerous condition. In contrast to routine cervical screening, which is undoubtedly effective, we should always remain vigilant about atypical situations in individual patients.