Introduction
Globally, cervical cancer is responsible for a significant number of human mortalities. Approximately 0.54 million new cervical cancer cases and 0.25 million cervical cancer-related deaths were reported in 2012 [1]. Cervical cancer is also known as the ‘disease of disparity’ owing to its inconsistent incidence in high- and low-income countries [2]. As per the estimates, cervical is the fourth most prevalent type of cancer worldwide, and it is believed that its incidence will increase significantly in the next decade [3]. Treatment of cervical cancer generally involves surgery and subsequent chemotherapy [4]. Nonetheless, the currently used chemotherapy has adverse effects and is less efficacious [5]. Therefore, the development of cervical cancer chemotherapy with no or negligible side effects is needed. Triterpenes constitute a vast and diverse group of chemical scaffolds generally synthesised by plants [6]. However, some bacteria have also been reported to biosynthesise simple triterpenes, such as hopene [7]. Approximately 20,000 triterpenes have been discovered so far, and many more await discovery. Despite the remarkable commercial applicability of triterpenes, the majority of the natural triterpenoids remain largely untapped [8]. Triterpenes have shown extraordinary potential to be used as drugs for the management of diseases as severe as cancer [9]. Camelliol C is an important triterpenoid [10]. The anticancer activity of camelliol C has not been explored against human cervical cancer cells.
This study was therefore undertaken to investigate the anticancer effects of camelliol C against human cervical cancer cells and to decipher the underlying molecular mechanisms.
Material and methods
Cell lines and culture conditions
The normal NCEC line and cervical cancer cell lines (Csdki, HeLa, C33A, and siHa) were purchased from ATCC, USA and cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, USA) medium with 10% FBS at 37°C in a humidified atmosphere of 5% CO2.
Cell viability assay
MTT assay was performed in order to determine the proliferation of normal and cervical cancer cells after being treated with different doses of camelliol C in a 96-well plate. The treatments were applied for 24 h at 37°C. After 24 h, the MTT reagent was added to the samples and incubation at 37°C was prolonged for a further 4 h. The formazan product thus formed was suspended using the DMSO solution. The samples were red for the absorbance measurements at 450 nm. The OD450 values were used for inferring the proliferation rates of the culture samples.
DAPI staining assay
To examine the cervical cancer cell apoptosis after their treatment with 0, 5, 10, or 20 µM camelliol C in six-well plates at 37°C for 24 h, the harvesting of cancer cells was performed through the centrifugation at 5000 rpm. The cells were than subjected to staining with DAPI for 10 min. The cells collected were fixed with methanol after being washed with PBS buffer. Afterwards, the cells were examined for the fluorescence measurements using a fluorescence microscope.
Annexin V-FITC/PI staining
The HeLa cells were cultured in six-well plates at a density of 3 × 105 cells per well, as described previously [11]. The cells were then incubated for 24 h and treated with different doses of camelliol C for 48 h. The cells were then collected by trypsinisation. Subsequently, these cells were stained with Annexin V-FITC and PI for 25 min in the dark at 37°C. Finally, a Beckman Coulter EPICS-XL flow cytometer was used for the determination of the percentage of apoptotic cells.
Cell cycle analysis
After treatment with 0, 5, 10, or 20 µM camelliol C in 12-well plates at 37°C for 24 h, the cell cultures were centrifuged, and the harvested cells were fixed with 4% formaldehyde. The cells were mixed with propidium iodide solution. Following this, a flow cytometer was used to investigate the phase distribution of the cancer cells.
Migration and invasion assay
A transwell chamber without or with Matrigel coating was used to assess, respectively, the migration and invasion of transfected cervical cancer cells. Briefly, 100 µl of cell culture containing 6000 cells was added to the upper chamber of the transwell, and lower chamber was given 750 µl of DMEM medium supplemented with 10% FBS. After 48 h incubation at 37oC/5%CO2, cells from the surface of membrane’s upper side were removed carefully with cotton swabs while those that stuck to the lower side of membrane were fixed with 70% ethyl alcohol and stained with 0.1% crystal violet. A light microscope (× 100) was used for visualisation of cells, and photographs were taken. At least seven random fields were used for the counting of migratory or invasive cells.
Estimation of ROS and MMP
The mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) quantification was done by DiOC6 (1 µmol/l) and dihydrofluorescein diacetate (10 µM), respectively, by flow cytometrically and are presented in the form of bar diagrams. The procedure was repeated three times.
Western blotting
After washing the camelliol C-treated cervical cancer cells with PBS, the cells were lysed using RIPA buffer and centrifuged to collect the supernatant. BCA assay was utilised to determine the protein content of the cell extracts. Subsequently, 20 µg of protein was subjected to separation on SDS-PAGE and then shifted to PVDF. Next, non-fat milk was used for blocking, and then the membranes were incubated with primary antibodies for 3.5 h at 37°C and then with secondary antibody at 25°C. Finally, an ECL-chemiluminescent kit was used to observe the protein bands of interest.
Statistical analysis
The experiments were performed in triplicate and expressed as mean ± SD. For statistical analysis, one-way ANOVA followed by Turkeys test were performed using SPSS software. The experiments were performed in triplicate and p < 0.05 was taken as a statistically significant difference.
Results
Camelliol C suppresses the viability of cervical cancer cells
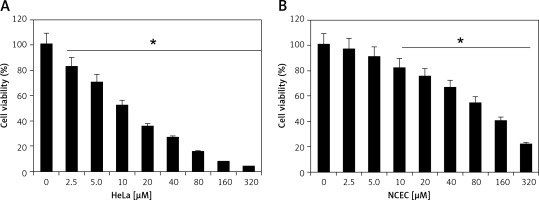
Camelliol C exerts inhibitory effects on the viability of the cervical cancer cells, as ascertained by MTT assay. The normal NCEC and four different types of cervical cancer cell lines (Caski, HeLa, C33A, and siHa) were treated with 0–320 µM concentrations of camelliol C for 24 h, and the proliferation rates of cells were determined. It was found that the viability of all the cervical cancer cells decreased proportionally with the increasing doses of camelliol C with IC50 ranging from 10 to 20 µM (Table I, Figure 1 A). Surprisingly, the effects of camelliol C on the normal cells were less severe, as shown by the IC50 of around 90 µM (Figure 1 B). Because the lowest of IC50 of 10 µM was observed against the HeLa cells, this cell line was selected for further investigation.
Camelliol C promotes ROS-mediated cell death in cervical cancer cells
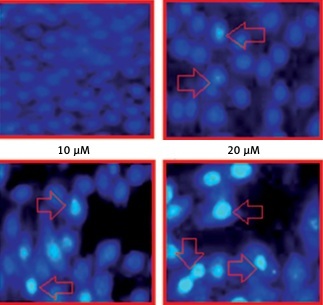
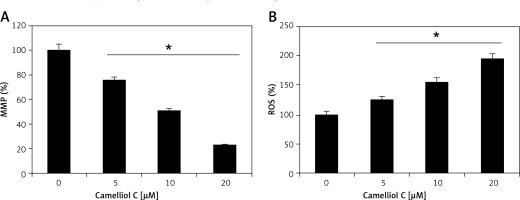
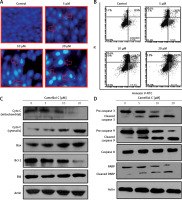
The results of fluorescence microscopy of DAPI stained HeLa cells revealed that the anti-proliferative effects of camelliol C on the proliferation of human cervical cancer cells were mainly due to the initiation of apoptosis in the HeLa cervical cancer cells, as shown by the increase in the nuclear fragmentation of the HeLa cells (Figure 2 A). The extent of apoptosis induced by camelliol C was determined by annexin V/PI staining. The percentage of apoptosis increased from 6.5% to 24.2% from control to 20 µM camelliol C (Figure 2 B). That camelliol C induces apoptosis was also further validated by investigating the effects of the expression of the apoptosis-related proteins. The expression of cytochrome c and Bax increased while the protein levels of Bcl-2 showed a dose dependent decrease. However, Bid expression remained almost unaltered at all concentrations of camelliol C (Figure 2 C). The results showed a remarkable increase in the cleavage of caspsase-3 and -9 as well as PARP in camelliol C-treated HeLa cells. The expression of caspase-8 remained more or less constant (Figure 2 D). The results also showed that camelliol C increased ROS levels and concomitantly decreased MMP levels in HeLa cells (Figures 3 A, B) suggesting that camelliol imitates ROS mediated cell death in HeLa cells.
Figure 2
Camelliol C induces apoptosis in cervical cancer cells. DAPI (A) and annexin V/PI (B) staining of camelliol C-treated HeLa cells, showing induction of apoptosis. Western blots of Cyto C, Bax, Bcl-2, Bid (C), and caspase-3, -9, and -8 and PARP (D). The experiments were performed in triplicate
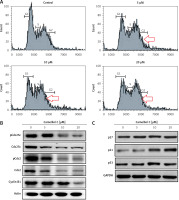
Camelliol C promotes G2/M arrest of cervical cancer cells
Flow cytometry analysis of the camelliol C-treated cells showed that inhibition of the HeLa cell proliferation was not only because of the apoptosis but also because of the G2/M cell cycle arrest. This was evident from the increase in the percentage of the G2/M phase cells with increased dosage of camelliol C (Figure 4 A). The initiation of the G2/M arrest was also linked with alteration in the expression of certain proteins, as revealed by western blotting. The phosphorylation of Cdc25c and cdc2 was considerably decreased. Additionally, the protein levels of cyclin B1 were also depleted (Figure 4 C). The expression of p27 and p53 showed no alteration, and that of p21 showed a constant increase (Figure 4 D).
Figure 4
Camelliol C induces G2/M cell cycle arrest in cervical cancer cells. A – Flow cytometry showing the effect of camelliol C on the cell cycle distribution of HeLa cells. B – Western blots showing the effect of camelliol C on Cdc25 c and Cdc2 phosphorylation. C – Western blots showing the effect of camelliol C on p27, p21, and p53 expression. The experiments were performed in triplicate
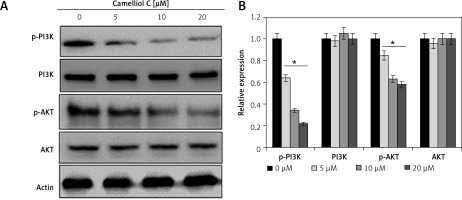
Camelliol C blocks PI3K/AKT signalling
The impact of camelliol C was also evaluated on the PI3K/AKT signalling pathway. It was found that the protein levels of p-AKT and p-PI3K declined significantly, and in a concentration-dependent manner (Figure 5). Nonetheless, there was no apparent effect on the total PI3K and AKT.
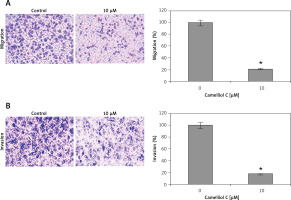
Camelliol C suppresses migration and invasion of HeLa cells
Determination of the effects of camelliol C on the migration and invasion of the human HELA cervical cancer cells was done by transwell assays. The results revealed that the camelliol C suppressed the migration of the HELA cell concentration dependently (Figure 6 A). The effects of camelliol C were also determined on HELA cell invasion, and the results were similar to those for cell migration (Figure 6 B).
Discussion
Plants have to adapt to harsh and changing environmental conditions, and over the course of evolution they have learned to defend themselves by synthesising a wide array of molecules, including triterpenoids. Triterpenoids constitute a large and diverse group of plant-derived compounds with enormous pharmacological potential [12]. They are ubiquitously found across the kingdom Plantae. These plant-derived metabolites have a wide array of bioactivities in plants, such as anticancer and anti-microbial [13]. Accordingly, active research is ongoing to examine the anticancer effects of triterpenoids against different human cancers. This study was undertaken to evaluate the anticancer effects of camelliol C, a naturally occurring triterpenoid of plant origin against human cervical cancer cells. The cell proliferation assay showed significant inhibition of cervical cancer cell growth upon camelliol C treatment. Previous studies have shown that triterpenoids have the potential to trigger apoptosis in different cancer cells [14]. More precisely, the plant-derived triterpenoid friedelin has been shown to suppress the growth of lung cancer cells via induction of apoptosis [15]. The expression of cleaved caspase-3 and -9, as well as that of cleaved PARP, was remarkably increased. Additionally, the Bax/Bcl-2 ratio was also increased, which is an important indicator of apoptosis [16]. Apoptosis plays a key role in eliminating the defective cells, and thus drugs that promote apoptosis are currently being studied extensively [17]. We also investigated the effects of camelliol C on the HeLa cell distribution and found that camelliol C induces G2/M arrest of HeLa cells and also blocked the phosphorylation of CCdc2 and CCdc25c. These findings may explain for the low IC50 of camelliol C against the HeLa cells. Several triterpenoids have also been shown to induce cell cycle arrest of cancer cells, which is in agreement with the present study [18]. This study also examined the effects of camelliol C on migration and invasion of the HeLa cells. It was found that camelliol C suppressed the migration and invasion of the HeLa cells. These findings are in agreement with a previous study in which a triterpenoid, ursolic acid, was found to suppress the migration and invasion of cancer cells [18]. Aberrant activation of the PI3K-Akt pathway has been shown to be involved in the development and progression of different cancer types. PI3K was first identified as an enzymatic activity associated with the Rous sarcoma pp60v-src protein and the polyoma middle T antigen, which is essential for the transforming activity of these oncogenes, and Akt was also found to be a viral oncogene [19, 20]. Studies have shown that PI3K/AKT may prove to be an optimal target for anticancer drugs [21]. Owing to the importance of this pathway, drugs targeted to inhibit this pathway may prove essential in the management of this pathway [19, 22]. Herein, we observed that camelliol C blocks the both the PI3K/AKT signalling pathway. Hence, more studies are required to establish camelliol C as a lead molecule for the development of chemotherapy for cervical cancer. Although this study evaluated the anticancer effects of camelliol C under in vitro conditions, this study will definitely pave the way for its in vivo evaluation.
In conclusion, the results of the present study show that camelliol C inhibited the growth of human cervical cancer cells via induction of apoptosis and G2/M cell cycle arrest. Camelliol C also suppresses the migration and invasion of cervical cancer via blocking the PI3K/AKT pathway. These findings point towards the potential of camelliol C as a lead molecule for the treatment of cervical cancer.