Nasopharyngeal carcinoma (NPC) is an ascending squamous cell carcinoma located in the retronasal cavity. Due to the occult site, many patients present with synchronous distant metastases at initial diagnosis, with the bone, lung, and liver being the most common sites. Studies have shown that liver metastases may be an independent negative prognostic factor compared with bone or lung metastases [1]. NPC patients with liver metastases have a very short survival time, with a median survival time of only about 18.0 months [2], and different treatment regimens have different survival rates [3]. Therefore, how to choose a treatment regimen to maximize the benefit of patients with liver metastases of NPC is an urgent problem to be solved. Systemic chemotherapy is an important treatment for advanced NPC with recurrence or distant metastasis, but its efficacy is limited. Immunotherapy combined with chemotherapy has been reported to improve the effective rate. For example, in a phase 3 study, camrelizumab plus gemcitabine and cisplatin (GP) extended the progression-free survival (PFS) significantly compared with GP chemotherapy (median 9.7 vs. 6.9 months; HR = 0.54; 95% CI: 0.39 to 0.76; p = 0.0002) [4].
In recent years, immunotherapy represented by programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) immune checkpoint inhibitors has changed the status of anti-tumor therapy for NPC [5, 6]. Studies have shown that anti-PD-1 inhibitors have good antitumor activity and good tolerability in patients with recurrent or metastatic nasopharyngeal carcinoma (R/M NPC) [4], which may lead to long-term survival benefits. However, the observed overall survival (OS) benefit was significantly lower in R/M patients with liver metastases than in R/M patients without liver metastases (13.3 vs. 23.8 months, p = 0.006) [7]. Tislelizumab (TIS) is an investigational humanized IgG4 monoclonal antibody with high affinity and binding specificity for PD-1. TIS was engineered to minimize binding to FcγR on macrophages in order to limit antibody-dependent phagocytosis. A phase 3 study (NCT03924986) introduced TIS plus GP as first-line therapy for R/M NPC. An independent review committee assessed that the median PFS (hazard ratio = 0.54 (0.38, 0.76); 9.6 vs. 7.4 months) and objective response rate (ORR; 69.5% vs. 55.3%) were significantly better than those of placebo plus GP [8]. In this case report, we describe an NPC patient with liver metastasis and discuss the treatment strategies.
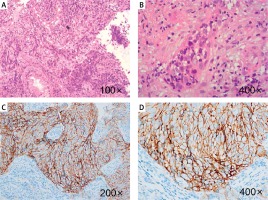
A 50-year-old man was hospitalized due to “bloody snot for 5 months, headache, blurred vision, and bilateral cervical lymphadenopathy for 3 months” in December 2020. The patient had tinnitus, hearing loss, right eyelid ptosis, and numbness on the right side. Physical examination showed multiple enlarged lymph nodes on both sides of the neck. Pathological biopsy combined with immunohistochemical results showed that it was consistent with (nasopharyngeal) nonkeratinizing undifferentiated NPC (Figure 1). The value of Epstein–Barr virus (EBV) DNA was 1.12 × 104 copies/ml. Positron emission tomography-computed tomography (PET-CT) showed that NPC invaded the bilateral retropharynx and superficial area II–IV of the right neck, several lesions in the left lobe of the liver and lymph nodes in the head of the pancreas were active, and metastasis was considered. In summary, the patient was diagnosed with nonkeratinizing undifferentiated NPC with liver and lymph node metastases in the head of the pancreas, stage IVb (cT4, cN2, cM1) based on the criteria of the 8th American Joint Committee on Cancer. Moreover, the expression of PD-L1 was high (approximately 80% positive, Figure 1).
Figure 1
Pathologic diagnosis: nonkeratinizing undifferentiated nasopharyngeal carcinoma. A – Tumor cells were arranged in irregular island-like, non-adhesive sheets or beams. Cancer nests were mixed with different numbers of lymphocytes and plasma cells. B – The tumor cells were large, syncytial-like, with ill-defined cell boundaries, round or oval vesicular nuclei, and a large central nucleolus. C, D – Immunohistochemical staining for PD-L1 expression (approximately 80% positive)
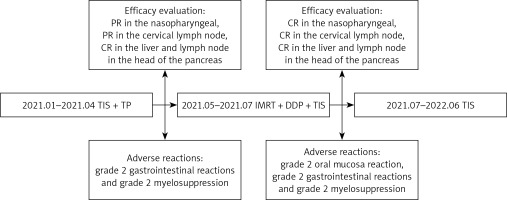
The patient received a TP regimen (nab-paclitaxel 260 mg/m2 + cisplatin 80 mg/m2) combined with PD-1 inhibitor (TIS 200 mg) every 3 weeks for five cycles from January to April 2021. Efficacy evaluation (Figure 2): partial response (PR) in the nasopharyngeal, PR in the cervical lymph node, complete response (CR) in the liver and lymph node in the head of the pancreas. During the treatment period, the patient developed grade 2 gastrointestinal reactions and grade 2 myelosuppression. The EBV DNA decreased from 1.12 × 104 copies/ml to less than 500 copies/ml (negative, according to the standard of monitoring instruments in our hospital) after three cycles of therapy. Subsequently, the patient received intensity-modulated radiation therapy (IMRT) of nasopharyngeal and cervical lymph nodes on May 19, 2021. Prescribed doses were 69.96 Gy to the gross tumor volume of the nasopharynx, 69.96 Gy to gross cervical lymph nodes, 62 Gy to the prophylactic radiation area of the primary lesion, and 54 Gy to bilateral cervical fields in 33 fractions, five times per week. During IMRT treatment, the patient was treated with 200 mg of TIS on day 1 plus 80 mg/m2 of cisplatin for one cycle. Efficacy evaluation after radiotherapy (Figures 2–4): CR in the nasopharyngeal, CR in the cervical lymph node, and CR in the liver and lymph node in the head of the pancreas. Adverse reactions: grade 2 oral mucosa reaction, grade 2 gastrointestinal reactions, and grade 2 myelosuppression. The patient was placed on maintenance therapy with TIS (200 mg every 3 weeks) from July 31, 2021 to June 30, 2022. The patient was reexamined every 3 months, and the last time the efficacy was evaluated was April 22, 2022. The patient still maintained CR without adverse reactions. The complete treatment history is shown in Figure 5.
Figure 2
Magnetic resonance imaging (MRI) showed the primary focus of NPC, lymph node metastasis in the neck, and liver and lymph node metastases in the head of the pancreas. A–D – Images of each lesion before treatment. E–H – Images of each lesion after five courses of chemotherapy combined with immunotherapy. I–L – Images of each lesion after chemoradiotherapy combined with immunotherapy
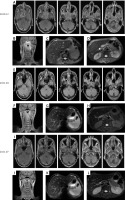
Figure 3
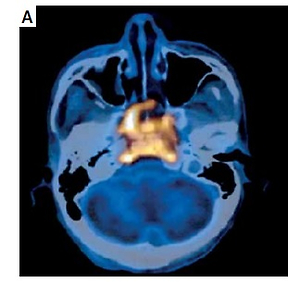
PET-CT showed the primary focus of NPC, lymph node metastasis in the neck, and liver and lymph node metastases in the head of the pancreas. A – Primary lesion before treatment. B – Before treatment, the largest cervical lymph node was 1.9 cm in short diameter. C – Before treatment, the largest liver metastasis was 1.4 cm in short diameter. D – Before treatment, the lymph node metastases in the head of the pancreas were 2.4 cm in short diameter. E–H – After treatment, the primary lesion, lymph node metastasis in the neck, and liver and lymph node metastases in the head of the pancreas completely disappeared
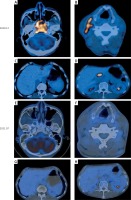
Figure 4
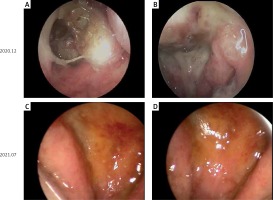
Nasopharyngeal endoscopy showed the primary focus of NPC. A – Watery discharge is seen in the right common nasal passage. B – On the right side of the pharyngeal recess, there is a raised mass with a non-smooth surface. C – Nasopharyngeal changes after radiotherapy and chemotherapy; nasal mucosa showing slight hyperemia. D – There was an abundant purulent secretion in the nasopharynx, and no obvious new organism was found
For patients with newly diagnosed metastatic NPC, 4–6 cycles of a two- or three-drug platinum-based combination regimen are still recommended. Studies have shown that systematic chemotherapy combined with radiotherapy has a significant survival advantage compared with chemotherapy alone [9].
GP and TP regimens were recommended as first-line treatment regimens for recurrent and metastatic NPC by the Chinese Society of Clinical Oncology in 2021, but the adverse reactions of TP regimen were significantly lighter than those of the GP regimen. In addition, more and more clinical trials [10, 11] have confirmed the safety and effectiveness of two-drug or three-drug combinations containing paclitaxel in metastatic NPC. Therefore, the TP scheme was adopted in this case. Of note, we chose nab-paclitaxel. Nab-paclitaxel is based on nanotechnology platforms without solvent-targeted chemotherapy drugs, solving the problem of Taxol solubility and solution stability. Moreover, it has the characteristics of attenuated synergies. At the same time, compared with the traditional paclitaxel, nab-paclitaxel has better tumor selectivity and lower cytotoxicity in normal tissues, leading to improved drug tolerance.
When patients with metastatic NPC were negative or positive for liver metastasis, the 3-year OS was 33.7% and 11.4%, respectively [2], whereas the 3-year survival rate of patients who achieved CR or PR after chemotherapy for liver metastases was 30.8% after primary tumor radiotherapy [1]. In order to improve the survival rate of patients, we attempted to find a new treatment, that is, on the basis of radiotherapy and chemotherapy combined with immunotherapy. Anti-PD-1 immunotherapy has become a treatment for R/M NPC, which can significantly improve the efficacy without increasing the toxicity. Research data on single-agent anti-PD-1 immunotherapy for R/M NPC showed that the ORR, durable response rate, and PFS of TIS [12] were higher than those of camrelizumab [13] and toripalimab [14]. Therefore, we suggest that patients should be treated with immunotherapy in addition to chemotherapy, and we chose TIS for our patient.
In patients with NPC and liver metastases, the CR and PR after platinum-based chemotherapy with or without radiotherapy were 5.4% and 64.8%, respectively [1]. In the present case, after three cycles of chemotherapy combined with immunotherapy, the EBV DNA decreased from 1.12 × 104 copies/ml to less than 500 copies/ml. Moreover, the liver and lymph node metastases in the head of the pancreas achieved CR after five courses of therapy. After radiotherapy combined with immunotherapy, the nasopharyngeal and cervical lymph nodes achieved CR. Our patient not only achieved a good curative effect, but also the adverse reactions were controllable. Generally, patients will have many common adverse reactions after chemotherapy, such as nausea/vomiting, bone marrow suppression, and liver damage [15]. In our case, the patient only experienced grade 2 gastrointestinal reactions and grade 2 bone marrow suppression during the period after chemotherapy combined with immunotherapy. In addition to the patient’s good tolerance, this is also due to the low toxicity of nab-paclitaxel. Radiotherapy also has many adverse reactions, such as skin reaction and mucositis [15]. In our case, the patient only experienced grade 2 oral mucosal reactions, grade 2 bone marrow suppression, and grade 2 gastrointestinal reactions after concurrent radiotherapy and chemotherapy combined with immunotherapy. In the 2020 and 2021 NCCN and ESMO guidelines for metastatic NPC, radiotherapy is recommended after chemotherapy, and it is not clearly indicated whether chemotherapy is required during radiotherapy. In our case, the liver and lymph node metastases in the head of the pancreas achieved CR after five cycles of chemotherapy combined with immunotherapy; the nasopharyngeal and cervical lymph nodes achieved PR; and the patient developed a grade 2 oral mucosa reaction, grade 2 gastrointestinal reactions, and grade 2 myelosuppression after one cycle of concurrent chemoradiotherapy. Therefore, in the case of a good curative effect, we took into account the cumulative toxicity of platinum. Only one cycle of immunotherapy combined with chemotherapy was performed during radiotherapy to reduce the adverse reactions of the patient.
Studies have shown that there is a synergistic mechanism between chemotherapy or radiotherapy and immunotherapy. Chemotherapy can directly kill cells, promote the release and expression of EBV antigen, prevent the development of immune failure, and thus synergistically promote the anti-PD-1 immune regulatory effect [16]. Radiotherapy can not only kill tumors, but also change the immunophenotype of residual tumor cells, such as the upregulation of PD-L1 expression [17]. Therefore, considering the reported mechanism of action and the efficacy and safety of our case, chemoradiotherapy combined with immunotherapy is worth investigating for the treatment of metastatic NPC.
In conclusion, chemoradiotherapy combined with immunotherapy may improve the efficacy of treatment of NPC patients with liver metastases. Patients may maintain disease-free survival for a long time from this regimen. In addition, the safety profile of this regimen is manageable.