Obstructive sleep apnea-hypopnea syndrome (OSAHS) is an increasingly recognized health issue, which is characterized by recurrent hypoxemia, wakefulness, and fragmented sleep [1]. Considerable evidence indicates that OSAHS is relatively prevalent in patients with hypertension, especially resistant hypertension [2]. Hypertension combined with OSAHS has become a disease that seriously endangers human health. In the last years, several reports on the underlying mechanism by which OSAHS increases the blood pressure have been published, involving a diverse range of mechanisms including oxidative stress, inflammation, enhancement of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nerves [3].
Recent studies have confirmed that inflammation and oxidative stress are significantly increased in patients with OSAHS compared with those without OSAHS by detecting biomarkers [4]. Obviously, such biomarkers should be highly specific and sensitive, and can reflect the severity and treatment response of OSAHS [5]. Of the biomarkers that met the requirements, interleukin-6 (IL-6) and C-reactive protein (CRP) are well suited to indicate inflammation, while NADPH oxidase and malonaldehyde (MDA) are suitable indicators of oxidative stress. These four biomarkers are closely related to the pathogenesis of OSAHS [3].
Continuous positive airway pressure (CPAP) is recognized as the most effective therapy for patients suffering from OSAHS [6]. However, it still needs to be clarified whether CPAP is effective in treating hypertension in patients suffering from OSAHS. With regards to the mechanisms by which CPAP affects blood pressure, many previous studies have confirmed that CPAP reduces blood pressure by counteracting sympathetic activation and the renin-angiotensin-aldosterone system (RAAS) [7]. However, there are few studies on the relationship between oxidative stress, inflammation, and CPAP treatment in OSAHS patients with hypertension [8]. We hypothesized that CPAP might be efficacious in treating hypertension in patients with OSAHS, and designed this randomized controlled trial to test the conjecture. In addition, in this study, we explored the underlying mechanism whereby CPAP lowers blood pressure, which may provide theoretical support for CPAP as a convincing treatment in addition to drugs.
Methods
This study was a prospective, single-center, randomized, parallel-group trial. It was conducted in the Sleep Medicine Center of our hospital from June 2019 to June 2021. All enrolled patients between the ages of 18 and 80 had OSAHS (AHI ≥ 5) and hypertension (systolic diastolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg). Exclusion criteria were: patients with other known causes of secondary hypertension; severe hypertension; using CPAP; patients taking any anti-inflammatory drug and/or psychotropic agent. We fully communicated with the patients before the experiment and obtained written consent from all enrolled patients. The Research Protocol was discussed by multidisciplinary researchers and supported by the Ethics Committee of our hospital.
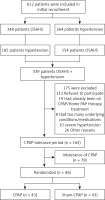
339 patients with a primary diagnosis of OSAHS and hypertension were sequentially enrolled in the PSG study, and 175 patients were subsequently excluded for various reasons. The remaining 164 participants initially underwent an acclimatization period (2 weeks, fixed pressure of 4 cm H2O) and a washout period (1 month). Then 86 OSAHS patients with hypertension were enrolled (Figure 1), including 47 males and 39 females. Baseline characteristics were documented, including age, body mass index (BMI), neck circumference, and waist circumference. These patients were then randomly divided into the CPAP treatment or sham CPAP control group without changing the original drug regimen for hypertension. All were subjected to the original pharmacological antihypertensive drug treatment combined with CPAP. Patients of the sham CPAP group received the fixed pressure of 4 cm H2O. Standard artificial pressure titration must be performed to determine the effective treatment pressure. The fixed CPAP pressure was used for the rest of the study in the treatment group. Before and after CPAP treatment, the differences in blood pressure changes (clinical, ambulatory, and home blood pressure) were compared between the two groups mentioned above. At the same time, the biomarkers including CRP, IL-6, NADPH oxidase, and MDA were measured. On average, CPAP was used for more than four hours per day, according to the preset CPAP prescriptions.
All patients underwent overnight PSG using the Philips Respironics, Alice 6 system. Data were obtained by manual scoring according to the criteria established by the American Academy of Sleep Medicine (AASM). We obtained blood pressure data (clinic, ambulatory and home blood pressure) at baseline and during follow-up. Under the premise of strictly adhering to the clinical guidelines, we used auto-titrating CPAP devices to complete CPAP treatment. All enrolled patients were followed up at 1 and 4 weeks, 8 and 12 weeks after initiation of treatment. As in our previous publications [9], we assessed CPAP adherence by weekly CPAP frequency and nightly CPAP time. Non-adherence refers to CPAP use less than 5 days/week or 4 h/night. After a 12-hour overnight fast, venous blood samples were taken from all patients at a fixed time (7 a.m. to 8 a.m.) before and after CPAP therapy. Then, the biomarkers (CRP, IL-6, NADPH oxidase and MDA) were determined according to the manufacturer’s instructions.
Statistical analysis
All data are presented as mean ± SD or as percentages. Pearson’s χ2 are test was used to analyze differences in blood pressure between the two groups, and Student’s t-test was used to analyze differences in biomarkers between the two groups. Correlations between the effect of CPAP on hypertension and its effect on biomarkers were verified using Pearson correlation analysis. All statistical work was done using SPSS version 25.0. P <0.05 was defined as statistically significant.
Results
Table I showed the effect of CPAP on ambulatory, clinic, and home blood pressure in patients with OSAHS combined with hypertension. No statistically significant difference existed in blood pressure between the two groups of patients before CPAP treatment (p > 0.05). In the sham CPAP group, there was no significant difference in blood pressure before and after CPAP treatment. Compared with the sham CPAP group (after therapy), CPAP treatment in the CPAP group resulted in significant improvements in night-time ambulatory systolic and diastolic blood pressure (p < 0.05), as well as 24-h ambulatory systolic and diastolic blood pressure (p < 0.05). In addition, compared with the sham CPAP group (after therapy), although the effect of CPAP on daytime dynamic systolic and diastolic blood pressure was weak, the results were still statistically significant. Throughout the experiment, we confirmed that the CPAP treatment significantly downregulates home systolic and diastolic blood pressure in the morning. Meanwhile, the effect of CPAP on home evening or clinic systolic/diastolic blood pressure was also statistically significant, although this effect was indeed weaker, especially for clinical systolic/diastolic blood pressure.
Table I
Treatment effects on blood pressure
| Variable | CPAP group | Sham CPAP group | ||
|---|---|---|---|---|
| Before therapy | After therapy | Before therapy | After therapy | |
| 24 h ambulatory blood pressure: | ||||
| 24 h systolic blood pressure | 132.23 ±10.4 | 130.51 ±10*#& | 131.95 ±10.63 | 131.79 ±10.69 |
| 24 h diastolic blood pressure | 87.14 ±8.55 | 84.3 ±8.5*#& | 87.28 ±8.53 | 86.98 ±8.29 |
| Daytime systolic blood pressure | 137.74 ±10.57 | 136.21 ±10.91*#& | 138.05 ±10.64 | 137.77 ±10.9 |
| Daytime diastolic blood pressure | 89.6 ±8.33 | 87.56 ±7.98*#& | 89.53 ±8.65 | 89.33 ±8.25 |
| Night-time systolic blood pressure | 126.21 ±10.59 | 122.16 ±10.09*#& | 125.88 ±10.6 | 125.56 ±10.44 |
| Night-time diastolic blood pressure | 79.07 ±8.55 | 74.4 ±7.63*#& | 79.26 ±8.64 | 79.21 ±8.55 |
| Home blood pressure evening: | ||||
| Systolic blood pressure | 137.79 ±10.68 | 135.19 ±10.32*#& | 138.02 ±10.61 | 137.79 ±10.59 |
| Diastolic blood pressure | 88.98 ±8.52 | 85.95 ±8.3*#& | 89.12 ±8.5 | 88.84 ±8.27 |
| Home blood pressure morning: | ||||
| Systolic blood pressure | 137.42 ±10.63 | 133.19 ±9.61*#& | 137.74 ±10.7 | 137.23 ±10.83 |
| Diastolic blood pressure | 89.98 ±8.48 | 84.93 ±7.73*#& | 89.67 ±8.75 | 89.53 ±8.34 |
| Clinic blood pressure: | ||||
| Systolic blood pressure | 137.44 ±10.68 | 136.7 ±10.15*#& | 137.6 ±10.8 | 137.19 ±10.26 |
| Diastolic blood pressure | 88.7 ±8.66 | 87.42 ±8.69*#& | 88.91 ±8.69 | 88.65 ±7.91 |
Table II shows the effect of CPAP on biomarkers reflecting inflammation and oxidative stress. Before CPAP treatment, no significant difference in these parameters was found between the two groups (p > 0.05). In the sham CPAP group, there was no significant difference in these biomarkers before and after CPAP treatment. After 12 weeks of CPAP treatment, compared with the CPAP group (before treatment) and sham CPAP group (after treatment), the patients in the CPAP group had significantly lower levels of CRP, IL-6, NADPH oxidase and MDA (p < 0.05). In addition, we used Pearson correlation analysis to confirm that the reduction of the night-time ambulatory systolic and diastolic blood pressure was significantly positively correlated with the reduction of IL-6, CRP, MDA and NADPH oxidase levels, respectively.
Table II
Treatment effects on inflammation biomarkers and oxidative stress biomarkers
| Variable | CPAP group | Sham CPAP group | ||
|---|---|---|---|---|
| Before therapy | After therapy | Before therapy | After therapy | |
| CRP [ng/ml] | 12.65 ±1.02 | 5.16 ±0.93*#& | 12.54 ±1.03 | 12.56 ±1.27 |
| IL-6 [pg/ml] | 43.46 ±3.08 | 18.8 ±1.39*#& | 43.69 ±2.79 | 43.63 ±2.7 |
| NADPH oxidase [U/ml] | 4.97 ±0.36 | 3.21 ±0.27*#& | 4.92 ±0.33 | 4.93 ±0.31 |
| MDA [nmol/ml] | 13.54 ±1.98 | 8.65 ±1.21*#& | 13.79 ±2.02 | 13.81 ±2.16 |
Discussion
In the current study, we investigated the effect of CPAP on clinical, ambulatory, and home blood pressure in patients with OSAS and hypertension. We found that appropriate use of CPAP has a significant antihypertensive effect on OSAHS patients complicated with hypertension, with a 1–5 mm Hg decline after 3 weeks. Additionally, we also found that the reduction in blood pressure due to CPAP was most obvious at night, and with the strongest impact on diastolic blood pressure values. This may be because the maintenance of diastolic blood pressure is mainly dependent on vasoconstriction, which is mainly affected by endothelin, nitric oxide, and catecholamines [10]. Also, the best effect of CPAP in reducing diastolic blood pressure occurs at night, probably because CPAP treatment of sleep apnea reduces the amplitude of changes in blood oxygen and thereby weakens sympathetic nerve excitability [11]. The effect of CPAP in lowering blood pressure at night lasts until waking up in the morning [11]. Thus, the direct effect of CPAP treatment results in lower nighttime and morning blood pressure, whereas daytime blood pressure regulation appears to be multifactorial [12].
We found in this study that CPAP significantly reduced inflammatory mediators (CRP, IL-6) and oxidative stress (NADPH oxidase, MDA) in the blood of patients with OSAHS and hypertension, which is consistent with previous literature reports [13]. Furthermore, we used Pearson correlation analysis to confirm that the therapeutic effect of CPAP on hypertension in OSAHS patients was significantly positively correlated with its inhibitory effect on oxidative stress and inflammation. Thus, this result confirms that nocturnal blood pressure reduction under CPAP is significantly positively associated with reduced inflammation and oxidative stress. In order to make up for the defects of this experiment, we will cooperate with other medical centers to further consolidate the results by expanding the sample size and extending the experimental time in the future.
In conclusion, the current research shows that CPAP has a good hypotensive effect on the night-time ambulatory blood pressure and morning blood pressure, especially on diastolic blood pressure. The inhibition of inflammation and oxidative stress by CPAP is likely to play an important role. Therefore, in addition to basic antihypertensive drugs, CPAP is an important intervention for patients with hypertension and OSAHS.