Introduction
The role of vitamins and minerals in human physiology has long been recognized. Over the decades, an extensive body of scientific literature has accumulated through clinical and experimental studies. Based on these data, and on more recent additional documentation, competent authorities, such as the European Food Safety Authority (EFSA) at the EU level, have recognized specific health claims for various micronutrients that can be used in consumer communication. As a fundamental principle, such claims may only be used for food products (naturally rich sources, fortified foods, and dietary supplements) that contain amounts considered nutritionally significant.
To obtain all the micronutrients essential for a variety of functions and metabolic processes critical to health and well-being – with the exception of vitamin D – the human body relies on dietary intake, particularly with a varied diet rich in nutrient-dense foods [1]. Individual factors – such as age, gender, body weight, physiological and pathological conditions that affect absorption or metabolism, dietary habits, and factors influencing bioavailability – can significantly affect nutrient requirements, absorption and utilization of vitamins and minerals, making it necessary to adjust intake. For these reasons too, dietary supplements and micronutrient-enriched foods are considered useful tools for filling nutrient gaps under certain conditions.
The maximum amounts of vitamins and minerals that may be contained in food supplements have been set by some health institutions in selected countries. These limits are regularly re-evaluated, mainly due to toxicological concerns about possible overconsumption.
However, it is important that such measures also fully recognize and protect the nutritional role and health functions of these micronutrients and that public health policies keep both the adequacy of micronutrient supply and their safety under control.
In this context, it should be noted that great attention is paid to the maximum levels of total daily intake without adverse effects for the general population: the Tolerable Upper Intake Levels (ULs), which are regularly reviewed. In particular, EFSA has recently reassessed intakes of vitamin B6 and vitamin D, highlighting that, based on available intake data, it is unlikely that the EU population exceeds the ULs, with the exception of regular users of food supplements containing high doses of vitamins [2, 3].
Rationale for the use of micronutrients in food supplements
The importance of ensuring adequate intake of vitamins and minerals, which are essential in most cases, and the observation of the functional and health effects that micronutrients exert at various levels in the human body are the fundamental factors that justify the attention of health organizations, clinicians, and nutritionists to these nutrients.
The intake levels of essential micronutrients are periodically reassessed based on scientific data by expert groups from international organizations such as the WHO/FAO [4] and EFSA, and national bodies such as the Italian Society of Human Nutrition in Italy [5] and ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail) in France. These reference values help evaluate population intake, ensuring adequate nutrient levels for health while identifying risks associated with deficiencies or excessive consumption.
The amounts necessary to define a product as a “source” or “rich” in a vitamin and/or mineral – based on providing e.g. nutritionally relevant amounts associated with recognized and authorized health effects – are in line with the population reference values. In the European Union, these amounts correspond to at least 15% of the Nutrient Reference Value (NRV) listed in Annex XIII of Regulation (EU) No. 1169/2011.
In the specific case of dietary supplements, which, according to Directive 2002/46/EC, are intended “to supplement the normal diet and which are concentrated sources of nutrients … in dose form, … designed to be taken in measured small unit quantities,” the nutritionally relevant amount of the micronutrient must be provided in the daily intake indicated on the label. According to some organizations, this amount should not be less than 15% of the NRV.
Maximum amounts allowed in food supplements: the European context
As previously mentioned, the focus of health institutions and research is not limited to ensuring an adequate intake of micronutrients. It also extends to the potential risk of excessive intake, particularly in specific population groups.
The ULs, established and periodically updated based on available evidence, represent the highest intake levels that certainly not pose adverse health effects.
To prevent the risk of excessive intake of one or more of these micronutrients due to the use of dietary supplements, Directive 2002/46/EC mandates the definition of maximum amounts of micronutrients that may be added to products in this category per daily portion. These limits are derived from both the ULs and habitual intake from other dietary sources.
To date, in the absence of harmonized EU-wide regulations, several countries, including Italy, France, Belgium, and Spain, have established national ULs for the presence of micronutrients in supplements. In some cases, these limits differ from one another, reflecting variations in reference data, dietary intake estimates from fortified foods, and differences in risk assessment approaches (Tables I, II). Italy has adopted rather conservative ULs for certain vitamins, such as D, E, and B6, whereas for others, such as B12, phosphorus, and iodine, the maximum levels are higher than those set in other countries.
Table I
Maximum levels of vitamins permitted in food supplements in some European countries
| Parameter | Italy1 | France2 | Belgium3 | Denmark4 | Poland5 | Ireland6 | Germany7 | Netherlands8 | UK9 | Slovenia10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin A – RE | µg (RE)/day | 1200 | 1000 | 1200 | 890 | 800 | 3000 | 3000 | 1200 | 1500 | |
| Vitamin A – β-carotene | mg/day | 7.5 | 7 | 7 | 7 | ||||||
| Vitamin D | µg/day | 50 | 50 | 75 | 95 | 50 | 100 | 100 | 75 | 20 | |
| Vitamin E | mg/day | 60 | 150 | 39 | 291 | 250 | 300 | 18 | 70 | ||
| Vitamin K | µg/day | 200 | Quantum satis | 210 | 919 | 200 | 80 | ||||
| Vitamin C | mg/day | 1000 | 1000 | 1000 | 1000 | 1000 | 2000 | 500 | |||
| Thiamine (B1) | mg/day | 25 | 4.2 | 100 | 7 | ||||||
| Riboflavin (B2) | mg/day | 25 | 4.8 | 40 | 8 | ||||||
| Niacin | mg/day | 54 | 8+450 | 10+54 | 10+891 | 16+830 | 10+900 | 90 | |||
| Vitamin B6 | mg/day | 10 | 12.5 | 6 | 10 | 18 | 25 | 20 | 21 | 10 | 8 |
| Folic acid | µg/day | 400 | 500 | 500 | 1000 | 600 | 1000 | 800 | 400 | ||
| Vitamin B12 | µg/day | 1000 | 3 | 100 | 15 | ||||||
| Biotin | mg/day | 450 | 450 | 500 | |||||||
| Pantothenic acid | mg/day | 18 | 18 | 10 | 30 | ||||||
1 Italian Ministry of Health; Apporti giornalieri di vitamine e minerali ammessi negli integratori alimentari; 2018;
2 French Agency for Food. Environmental and Occupational Health & Safety (ANSES); Avis de l’Agence nationale de sécurité sanitaire de l’alimentation. de l’environnement et du travail; 2024;
3 Arrete royal du 30 mai 2021 concernant la mise dans le commerce de nutriments et de denrées alimentaires auxquelles des nutriments ont été ajoutés (M.B. 11.VI.2021) 2024;
4 FOD Volksgezondheid. Veiligheid van de Voedselketen en Leefmilieu; Fødevarestyrelsen; Næringsstoffer og stoffer i kosttilskud;2024;
5 Główny Inspektorat Sanitarny; Zestawienie Uchwał Zespołu do spraw Suplementów Diety działającego przy Radzie Sanitarno-Epidemiologicznej; 2021;
Table II
Maximum levels of minerals permitted in food supplements in some European countries
| Parameter | Italy1 | France2 | Belgium3 | Denmark4 | Poland5 | Ireland6 | Germany7 | Netherlands8 | UK9 | Slovenia10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium | mg/day | 1200 | 800 | 1600 | 1327 | 1500 | 2500 | 1000 | |||
| Phosphorus | mg/day | 1200 | 750 | 1600 | 1727 | 450 | 1250 | ||||
| Magnesium | mg/day | 450 | 360 | 450 | 233 | 400 | 250 | 250 | 250 | ||
| Iron | mg/day | 30 | 14 | 45 | 37 | 20 | 45 | 18 | |||
| Zinc | mg/day | 15 | 15 | 22.5 | 11 | 15 | 250 | 25 | 15 | ||
| Copper | mg/day | 1 | 2 | 2 | 0.4 | 2 | 5 | 10 | 3 | ||
| Manganese | mg/day | 10 | 3.5 | 1 | 3.4 | 1.8 | 11 | 5 | |||
| Fluoride | mg/day | 4 | 3.5 | 1.7 | 5.4 | 3.5 | 8 | ||||
| Selenium | µg/day | 100 | 150 | 105 | 197 | 200 | 300 | 450 | 100 | ||
| Chromium | µg/day | 250 | 250 | 187.5 | 250 | 200 | 125 | ||||
| Molybdenum | µg/day | 100 | 300 | 225 | 541 | 350 | 700 | 150 | |||
| Iodine | µg/day | 225 | 150 | 225 | 134 | 150 | 600 | ||||
| Potassium | mg/day | 3000 | 6000 | 3000 | 1500 | ||||||
| Sodium | mg/day | 2.3 | |||||||||
| Silicon | mg/day | 700 | 350 | ||||||||
1 Italian Ministry of Health; Apporti giornalieri di vitamine e minerali ammessi negli integratori alimentari; 2018;
2 French Agency for Food. Environmental and Occupational Health & Safety (ANSES); Avis de l’Agence nationale de sécurité sanitaire de l’alimentation. de l’environnement et du travail; 2024;
3 Arrete royal du 30 mai 2021 concernant la mise dans le commerce de nutriments et de denrées alimentaires auxquelles des nutriments ont été ajoutés (M.B. 11.VI.2021) 2024;
4 FOD Volksgezondheid. Veiligheid van de Voedselketen en Leefmilieu; Fødevarestyrelsen; Næringsstoffer og stoffer i kosttilskud;2024;
5 Główny Inspektorat Sanitarny; Zestawienie Uchwał Zespołu do spraw Suplementów Diety działającego przy Radzie Sanitarno-Epidemiologicznej; 2021;
The ongoing attempt to harmonize these values at the EU level has encountered diverging viewpoints, likely due to the difficulty of balancing nutritional efficacy and safety.
A 2006 European Commission DG SANCO document [6] raised unresolved questions regarding whether limits should be set even for nutrients without a defined UL, whether it is necessary to regulate nutrients with an extremely low toxicity risk, and whether different limits should be established for supplements and fortified foods.
In 2008, AFSSA (now ANSES) analyzed various methodologies for determining maximum limits [7], taking into account dietary intake data and simulation models proposed by international institutes, including the ILSI (International Life Sciences Institute), the Danish Institute for Food and Veterinary Research, and the Federal Institute for Risk Assessment (BfR). For dietary supplements, it compared the values proposed by the ERNA (European Responsible Nutrition Alliance), EHPM (European Federation of Associations of Health Product Manufacturers), and BfR with those defined by France in 2006. This analysis resulted in varying public health protection scenarios, with differences in the identification of reference population groups used to define safety limits.
Recently, some countries have updated their national regulations. In 2024, the French Ministry of Agriculture proposed modifications to the maximum daily amounts of micronutrients allowed in supplements, based on recommendations from ANSES [8]. This revision considered the ULs defined by the EFSA and other authorities, consumption data from the Inca 3 (2014-2015) survey, and reported cases of adverse effects from nutrivigilance monitoring. Based on 165 reported cases, ANSES recommended lowering the maximum amounts of vitamin B6, zinc, selenium, and manganese to minimize the risk of exceeding ULs and suspending vitamin supplements for children under 3 years old, except under specific medical recommendation.
Industry trade associations have developed alternative proposals. A 2014 document suggested balancing the risk of deficiency and overdose using a Population Safety Index (PSI) [9]. This index is calculated as the ratio between the difference between the UL and maximum intake from food sources and the recommended daily dose. The PSI categorizes micronutrients into three groups: no known adverse effects, low risk of exceeding the UL, and high risk of excessive intake [10].
Another issue that needs adequate consideration in this context concerns the potential contribution of fortified foods to total micronutrient intake.
In the Netherlands, fortification with vitamin A, selenium, copper, and zinc is restricted to reconstituted or substitute foods, due to the narrow margin between recommended values and ULs [11]. In Belgium, on the other hand, fortification is allowed within the limits defined by the Superior Health Council, which were updated in 2021 [12].
In Italy, there is a lack of updated data on the contribution of different food products to the daily intake of micronutrients; however, a comparison between diet composition in 2005–2006 [13] and 2017–2020 unpublished data [5] shows no significant variations in the overall dietary pattern, but evidencing a trend of reduced intake of almost all examined compounds among women over 60 years old. Similarly, an analysis of food consumption patterns for key vitamin and mineral sources does not reveal significant changes that would justify lowering intake levels through specific food product categories (such as supplements) [14].
Overall, the regulation of dietary supplements and fortified foods remains a complex issue that requires balancing the need to achieve an adequate intake of micronutrients while preventing excessive intake, taking into account the diversity of dietary habits and reference populations.
On the one hand, supplementation should ensure an adequate supply of micronutrients, especially for individuals at the lower end of the intake distribution within the general population (i.e. those with the lowest consumption). On the other hand, it is important to ensure that those with the highest intakes (i.e. at the upper end of the distribution) do not exceed the limits considered safe, even if they choose to take supplements.
Furthermore, relying on population averages is not very useful for assessing adequacy and safety and may even lead to misleading conclusions.
Multivitamin/multimineral supplements are an apt example of this complexity. While some studies have found no significant health benefit of taking multivitamin supplements in the general population [15], these supplements can still play a critical role in addressing subclinical deficiencies that are often asymptomatic and difficult to diagnose, especially considering the costs associated with blood testing, and that occur in certain groups such as older adults or individuals in physiologically demanding situations such as pregnancy or lactation [16–18].
In the future, a more refined approach will include consideration of individual variability in micronutrient requirements, which may be influenced by genetic predisposition and other personal factors. This perspective, guided by the principles of “precision nutrition”, requires a paradigm shift from population-based recommendations to personalized nutritional interventions tailored to individual needs [19]. On the other hand, it is clear that such a transition would be quite complex to manage from a regulatory standpoint, due to the intrinsic difficulty of defining personalized thresholds in a regulatory context [20].
The approach to be taken in the setting of minimum and maximum amounts of micronutrients in food supplements therefore remains controversial: The adoption of more or less precautionary criteria regarding the potential toxic effects of these compounds, taking into account the significant range of uncertainty that characterizes the available data on their intake from different food sources (natural foods, fortified foods, and dietary supplements), is reflected in the higher or lower amount of these nutrients that is considered permissible to add to their only legally controllable vehicle, dietary supplements.
The precautionary approach
Recently, a procedure has been proposed by the BfR to estimate the maximum amounts of micronutrients that can be added to food supplements following a rigorous methodology [21]. However, it is based on a precautionary toxicological approach that does not include the evaluation of nutritional aspects of these nutrients.
Firstly, the difference between the UL and 95th percentile of intake is divided into two equal parts, with one part allocated to fortified foods and the other to food supplements. The proposal of such 50:50 division does not appear to be based on sound scientific data [21].
Another issue concerns the definition of ULs proposed by the BfR, which is sometimes influenced by the selection of individuals with specific conditions. For example, in the case of potassium, the reference group, which consists of patients with renal failure, who must limit their intake, is not representative of the general population, for which potassium is essential for maintaining normal blood pressure levels.
The BfR also suggests applying an additional safety factor of 2 to safeguard against the risk of combined intake from multiple sources of the same micronutrient. Available data, on the other hand, indicate that supplement use is mostly occasional, making it unlikely that ULs would be exceeded for prolonged periods [22]. The systematic use of this additional safety factor therefore could have unfavorable consequences by compromising the potential physiological role of these products.
Similar criticisms emerge in the literature regarding the application of the classic U-shaped dose-response curve for micronutrients, which differentiates between deficiency and toxicity [23]. Moreover, some authors highlight the inadequacy of equating the risk of deficiency with that of excessive intake, since minimum intake levels are based on objective data, whereas ULs are derived from a precautionary approach, often influenced by scientific uncertainties [2, 3, 24].
Conceptually, it is worth noting that reducing the limits of micronutrients that can be added to supplements may indeed reduce the risk of excessive intake (which is already very low, given the highly precautionary method used to calculate these levels), but at the cost of a much more likely reduction, and in some cases an almost certain one, in the ability of supplements to provide physiologically sufficient amounts of the considered molecules.
This concern is particularly relevant in view of the increasing incidence of hypovitaminosis in various age groups. The incidence of more or less severe scurvy, for example, is increasing in both children [25] and older adults [18, 26]. Low vitamin D levels are common in adults and the elderly and may be associated with an increased incidence of dementia [27]. A recent study found an association between serum vitamin D levels and cardiovascular health in adolescents in the US, suggesting that supplementation is important in a particularly high-risk group of teenagers [28]. Adequate vitamin D supplementation is also particularly important in women of childbearing age. Insufficient vitamin D levels are associated with the risk of developing gestational diabetes during pregnancy, which can have serious consequences for both mother and child [29].
The importance of optimal micronutrient intake for long-term health is underscored by Ames’ so-called triage theory [30], which states that under conditions of micronutrient deficiency, the human body prioritizes available micronutrients for biological functions that are important for immediate survival, while long-term protective functions – such as antioxidant defense and DNA repair – may be temporarily downregulated. While this adaptive mechanism of “emergency allocation” preserves short-term viability, it can contribute to the development of chronic disease over time, even in the absence of obvious clinical deficiencies. Micronutrient intake that is merely sufficient to prevent immediate deficiency symptoms may be insufficient to maintain long-term health, especially in societies with increasing life expectancy, such as ours.
Reference population
A critical aspect of this process is the selection of the group of individuals to be used as a reference for estimating the maximum allowable micronutrient intake from dietary supplements.
Some countries, such as Denmark and Germany, suggest using the most sensitive population segment for each micronutrient and designating children aged 1–3 years as the reference group for most vitamins and some minerals [20, 31]. This approach is very cautious and ensures that no population group can reach the UL. However, there is a risk that the content of micronutrients in food supplements is excessively reduced to levels that are of little or no relevance for healthy adults, who are the reference population group for the entire Nutrition and Health Claims Regulation.
For the necessary safety assessments and the estimation of both nutritionally significant and useful vitamin and mineral intakes, it is essential to collect the most accurate possible data on vitamin and mineral intakes in the general population, using a rigorous methodology, in representative samples in each country.
Several arguments support the selection of the adult population as the reference group.
Firstly, for the communication of nutritional and health information on food products (and thus dietary supplements), the reference group is healthy adults.
The NRVs established in Annex XIII of Regulation (EU) No. 1169/2011, which define the minimum concentrations that can be declared in a food product (or a supplement), are already aligned with the Dietary Reference Values (DRVs) identified for the adult population.
Secondly, the consideration that choosing the pediatric population as the reference group, since they are at a higher risk of experiencing excessive intake levels, due to a diet that is naturally rich in specific nutrients and/or supplements and/or fortified foods not specifically designed for them, would inevitably lead to the definition of maximum allowable amounts for micronutrients that are negligible in terms of nutritional intake for the adult population.
The exclusion of early childhood from the assessment is supported by several EFSA documents.
In 2013, the nutritional requirements of infants and young children were subjected to a scientific evaluation [32], which led EFSA Panel experts to conclude that the nutritional requirements for all macro- and micronutrients vary by age, from birth to 36 months.
The need for a specific and targeted approach to all aspects of infant nutrition in the early years of life also emerges from the virtual issue published in the EFSA Journal in 2020: Foods for infants and young children [33].
In this regard, it is worth noting that some countries have already proposed the identification of maximum amounts of micronutrients that can be added to supplements, differentiated by age group, from infancy to adolescence and into adulthood (for example, Ireland [34]).
Similarly, it is important that the permitted intake levels refer to the healthy population, and not to subgroups of the population affected by specific diseases, which may require restricted intake of certain micronutrients.
For example, potassium intake, previously mentioned, must be significantly reduced in patients with renal failure, but there is no evidence of benefits associated with reducing its intake in foods and/or supplements intended for the general population. On the contrary, evidence suggests a loss of the beneficial effects associated with restoring adequate intake levels, for individuals who, for example, follow highly restrictive or selective diets.
It would therefore be much more effective to inform patients with relevant conditions to be cautious with all foods, including fortified foods and food supplements, containing the vitamin or mineral that they need to limit or avoid, without restricting the ability of the healthy population, who may be at risk of insufficient intake of specific micronutrients, from achieving beneficial intake levels that support optimal health.
The role of fortified or enriched foods
In this context, it is also worth further examining the role of fortified foods, the consumption of which theoretically contributes to total micronutrient intake.
In general, it is necessary to analyze the cases in which food groups are typically enriched with vitamins and/or minerals. A first distinction must be made for products where fortification is mandatory or strongly recommended, due to a widespread deficiency of specific micronutrients in the population. This is the case – albeit limited in EU countries – as shown by the updated 2021 EU register, for the fortification of cereal flours with folates (mandatory in Canada, the United States, South America, Australia, and the United Kingdom), or the addition of folates to breakfast cereals, widely implemented in Scandinavian countries, to prevent neural tube defects in newborns [35–37].
In these situations of widespread or generalized risk of inadequate intake, it is intuitively unlikely that fortified foods would contribute to an excessive toxicity risk for the population.
Further considerations apply to the fortification of foods consumed as substitutes for others, e.g. in special diets, where the fortification aims to provide the same micronutrient intake as the original food it is intended to replace.
Plant-based beverages (made from legumes and cereals) are often fortified with calcium, plant-based burgers with iron and vitamin B12, fruit juices with vitamin C: but in all these cases, the goal is simply to make the product more similar to the natural food it is intended to replace (namely milk, meat, and fresh fruit), including in terms of micronutrient content. These fortifications, therefore, do not increase the overall total intake of these micronutrients in the population. Rather, it could be concluded that they help to prevent inadequate intake that could result from the public being unaware of the differences in composition between the original foods and their substitutes.
Thus, the contribution of such fortifications to a potential excess intake of these micronutrients can theoretically be considered negligible.
Proposed method to estimate the maximum allowable levels for micronutrients in dietary supplements
In the European context, characterized by the predominance of a toxicological approach, the methodology to be used in defining maximum amounts of micronutrients that can be added to supplements should instead carefully consider essential aspects of a strictly nutritional nature, in line with the considerations discussed so far and the guidelines of current regulations.
A possible logical sequence for the calculation and establishment of maximum levels in food supplements, taking into account these objectives, is outlined in the flowchart shown in Figure 1 and includes the following steps for each micronutrient: assessment of the intake distribution in the general population (mean intake, 5th and 95th percentiles), review of the ULs established by the competent authority (EFSA in the EU), calculation of the difference between the ULs and the indicators of adequate intake and the 5th, 50th, and 95th percentiles of the distribution in the general population. In certain cases, an assessment of possible contributions of fortified foods may be appropriate.
Figure 1
Proposed sequence for the assessment of the maximum micronutrient amount that can be added to food supplements
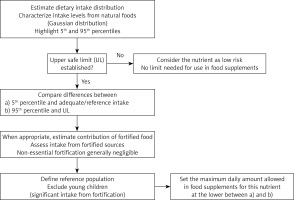
The evaluation of the difference between the values corresponding to the 5th, 50th, and 95th percentiles and the UL makes it possible to obtain the starting value for calculating the maximum amount for each micronutrient.
This limit must be set with the aim of preventing the UL from being exceeded with the total daily intake, even by people at the 95th percentile of the intake distribution. It is indeed very important to ensure that the permitted concentration in food supplements remains nutritionally significant so that also individuals at the 5th percentile of the intake distribution can obtain an adequate intake; to prioritize safety aspects, we suggest that the 95th percentile-UL distance should be used if it is smaller than the other one.
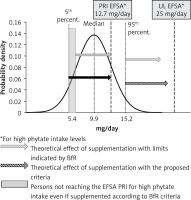
The different consequences of the proposed model and the BfR model can be examined using the example of zinc, whose intake in Italian adults and older people of both sexes corresponds approximately to the NRV for this mineral (10 mg/day) [13]. However, as the EFSA points out, zinc absorption can be significantly reduced by phytates, which occur naturally in many plant foods. For this reason, EFSA recommends a higher zinc intake for populations with diets rich in these compounds, up to 12.7 mg/day for women and up to 16.3 mg/day for men with the highest phytate intake [38]. With the maximum amount of 6.5 mg zinc in food supplements proposed by the BfR, most people who eat a diet rich in vegetables and thus phytates would not reach the recommended zinc levels even with food supplements.
In contrast, the proposed criteria would make it possible to achieve the recommended intake levels for the entire population (Figure 2). Interestingly, similar effects would be attained in other EU countries, considering the small differences in zinc intake among older women, with median values between 8.0 and 9.9 mg/day (9.9 in Italy).
Figure 2
Theoretical effects of zinc supplementation in elderly Italian women (65 years and above; data from the Italian National Food Consumption Survey INRAN-SCAI 2005–2006), considering the PRI proposed by EFSA for diets with high phytate intake levels [12, 36]. If the maximal amount of zinc in food supplements is calculated with the proposed criterion (filled black arrow), all women with low dietary intake, but above the 5th percentile, will reach PRI defined by the EFSA; a large part of them (grey area), on the other hand, will not reach PRI even if taking supplements if the maximal zinc content is calculated as suggested by the BfR. Striped arrows show that women with the highest intake (up to the 95th percentile) will not reach the limit set by the EFSA with either of the two supplementation levels considered
Conclusions
The definition of maximum daily amounts for vitamins and minerals in food supplements must allow consumers to obtain nutritionally significant amounts, so that supplementation has a real impact on the micronutrient balance and on the associated health effects, without reaching possibly toxic intake levels.
However, it is appropriate to compare the critical aspects that arise from two different assessments: the risk of providing insufficient amounts, which is likely when maximum limits are excessively low, and the possibility, on the other hand, of exceeding the UL, which is already regulated by a precautionary approach.
The predominance of a precautionary view, typical of the toxicological approach, would likely result in the adoption of overly restrictive criteria, which are poorly aligned with the recognized importance of micronutrients and the positive effects of an adequate intake, with the risk of limiting their intake even in population groups that would benefit the most.
Ultimately, it is important to establish the basic criteria for defining the optimal composition of supplements. If their purpose – as their name implies – is to supplement dietary intake (i.e. to compensate for potentially harmful nutritional deficiencies), then their formulation should provide nutritionally effective amounts of active ingredients, limited only by the threshold of apparent toxicity, to ensure that they provide a real benefit.
In the meantime, an approach that reconciles nutritional needs and safety aspects should be pursued.