Introduction
Hypertension remains one of the leading risk factors for cardiovascular morbidity and mortality worldwide [1]. While traditionally hypertension is attributed to genetic, dietary, and lifestyle factors, growing evidence highlights the role of the gut microbiota in the regulation of blood pressure (BP) [2]. The human gut harbors trillions of microorganisms that contribute to immune function, metabolic homeostasis, and vascular health. Dysbiosis – an imbalance in gut microbial composition – has been associated with increased inflammation, oxidative stress, and impaired short-chain fatty acid (SCFA) production, all of which may contribute to the development of hypertension and other cardiovascular diseases [3–5].
Results of recent clinical studies suggest that interventions targeting the gut microbiome, such as probiotics, prebiotics, and synbiotics, may offer novel therapeutic avenues for hypertension management. Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts, while prebiotics are substrates selectively utilized by host microorganisms to confer a health benefit. Synbiotics combine both, aiming to enhance microbial balance and functionality [3–6].
Mechanistically, the gut microbiota may influence blood pressure through modulation of SCFAs, regulation of the renin-angiotensin system, and maintenance of gut barrier integrity. These findings position the gut microbiota as a potential target for interventions in hypertension [3]. However, more robust clinical evidence is needed to confirm the therapeutic efficacy of such intervention [3]. Moreover, the optimal probiotic strain for antihypertensive effects remains to be determined.
This review explores current insights into the gut microbiome’s role in hypertension pathogenesis and evaluates the potential of microbiome-targeted therapies.
Gut microbiota – composition and functions in the human body
The human gut harbors an estimated 39 trillion microorganisms, which belong to three main domains: bacteria, archaea and eukaryotes, and viruses. According to some sources, the number of microorganisms in the intestines is approximately 1014, which is comparable to the number of nucleated cells in the human body. Microorganisms constituting the microbiota represent 1–3% of the total human body weight, i.e. approximately 2 kg [7–9]. These microorganisms include both commensal and symbiotic microorganisms, as well as those causing pathological conditions, including infectious diseases [7]. Metagenomic studies of the human microbiome have shown that there are 3.3 million genes in the human intestine, i.e. 150 times more genes than the human genome. Analysis of bacterial diversity has shown that approximately 1000 species of bacteria live in the human intestines [10]. Representatives of over 50 bacterial phyla are found in the human intestine [7]. The density of bacteria in individual intestinal parts varies and ranges from 103/ml in the duodenum to 1011/ml in the large intestine [7]. Colonization of the gastrointestinal tract by microorganisms begins during delivery. During individual growth, especially during the first 2–3 years of life, the intestinal microbiota undergoes the greatest modifications. Then, during life, the composition of the microbiota also changes [11]. The composition of the microbiota depends on the anatomical region of the human digestive tract, age, and numerous genetic and environmental factors (Figure 1) [12, 13].
Figure 1
Microbiota in various parts of the human digestive tract and selected factors influencing its diversity. Based on [12, 13]
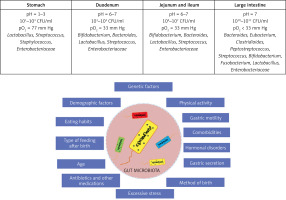
The predominant representatives of the gut microbiota are Firmicutes and Bacteroidetes, which constitute 90% of all gut bacteria. The phyla Proteobacteria, Actinobacteria and Fusobacteria are present in smaller amounts, although the qualitative and quantitative composition of bacteria in individual sections of the digestive tract differs depending on the prevailing conditions and is individually variable (Figure 1) [12, 13]. Although there is a wide range of inter-individual variability, some researchers suggest that most people’s gut microbiome can be assigned to one of three variants or “enterotypes” based on the dominant bacterial genera (Bacteroides, Prevotella or Ruminococcus) [14]. Factors with a strong effect leading to changes in the gut microbiota include changes in eating habits. Clinical studies have observed that changing a high-fat, low-fiber diet to a low-fat, high-fiber diet causes significant changes in the gut microbiota within 24 h. Moreover, diet type was also associated with enterotype, as people consuming a diet high in animal fats were found to be more likely to have a Bacteroides-dominant enterotype, while a diet high in carbohydrates was associated with a Prevotella-dominant enterotype [15].
The gut microbiota, among other functions, produces a variety of substances (Figure 2) and performs many functions in the human body, which can be placed in three main categories [16, 17].
Figure 2
Enzymes and chemical compounds produced by bacteria of the intestinal microbiota. Based on [16, 17]
SCFA – short-chain fatty acids, LCFA – long-chain fatty acids, TMA – trimethylamine, GABA – gamma-aminobutyric acid.
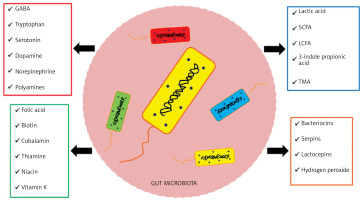
The trophic function involves the influence on differentiation and growth of the epithelium, leading to maintaining the integrity of the intestinal barrier and providing enterocytes with energy by producing SCFAs. The metabolic function involves, among other aspects, the breakdown of food residues to produce SCFAs and production of B vitamins and vitamin K2. The protective function is to act a barrier against intestinal colonization by pathogenic bacteria through inhibition of their development by peroxide and bacteriocins and by competing for nutritional requirements and places of colonization [18].
In recent years, a molecular biology technique has been developed that enables quantitative analysis of the composition of the intestinal microbiome [14]. The most widely used technique is based on the use of DNA, amplification of the 16S rRNA gene (rRNA) by polymerase chain reaction, and subsequent sequencing of its hypervariable regions. Within the 16S rRNA gene, encoding a component of the small ribosomal subunit, there are 9 hypervariable regions (V1-V9) that differ among bacterial species. After comparing their sequences with the data regarding the above RNA sequences from previously completed analyses, it is possible to identify individual bacterial strains in the tested sample. The data obtained in this way is then used to determine the diversity and composition of the gut microbiome [7]. To assess the composition and diversity of the gut microbiota, the Chao 1 index (a measure of bacterial richness, i.e. the number of species in the examined sample) and the Shannon index (a measure of diversity; when there is only one species, the value of this indicator is zero) are calculated [7].
Disturbances in the composition of the gut microbiome are called dysbiosis [18]. Dysbiosis is characterized by: 1) a decrease in the number of beneficial bacteria, 2) an increase in the number of potentially pathogenic bacteria, 3) a decrease in the number of commensal and symbiotic bacteria, 4) an increased Firmicutes (Gram-positive)/Bacteroidetes (Gram-negative) ratio and 5) loss of microbiota diversity (decreased Shannon index) [18, 19].
The results of clinical studies indicate that dysbiosis is involved in the pathogenesis of many diseases, including: inflammatory bowel diseases, obesity, diabetes, colorectal cancer, liver diseases, kidney diseases, cardiovascular diseases and neurological diseases [20].
Gut microbiota in patients with arterial hypertension
The results of clinical studies indicate that the gut microbiota of patients with hypertension differs in terms of diversity from the gut microbiota of normotensive subjects [21]. In the study by Li et al., the composition of the gut microbiota was analyzed in patients with untreated (n = 63) and treated (n = 104) hypertension and dyslipidemia (n = 26) and compared with healthy subjects (n = 42). Stool samples were tested using 16s rRNA gene sequencing. Differences in the composition of the gut microbiota were found between studied groups of patients. Bacteroides and Faecalibacterium genera predominated in the gut microbiota of healthy subjetcs, while in patients with untreated hypertension, bacteria of the Blautia, Bacteroides and Faecalibacterium genera predominated, and Prevotella predominated in patients with treated hypertension. The intestinal microbiota of patients with dyslipidemia, similarly to controls, contained mainly Bacteroides and Faecalibacterium [22]. Dysbiosis of the gut microbiota was also demonstrated by Dan et al. in a study involving 62 patients with hypertension and 67 subjects with normal blood pressure. The assessment of the gut microbiota was carried out using 16s rRNA gene sequencing from the subjects’ stool samples. Significant differences in the gut microbiota were found between the study groups. Eighteen genera (Acetobacteroides, Alistipes, Bacteroides, Barnesiella, Butyricimonas, Christensenella, Clostridium sensu stricto, Cosenzaea, Desulfovibrio, Dialister, Eisenbergiella, Faecalitalea, Megasphaera, Microvirgula, Mitsuokella, Parabacteroides, Proteiniborus and Terrisporobacter) showed higher abundance in hypertensive patients, while 36 genera (Acetobacteroides, Acidaminobacter, Adlercreutzia, Anaerotruncus, Asteroleplasma, Bulleidia, Cellulosilyticum, Clostridium III, Clostridium IV, Clostridioides XlVa, Coprobacter, Enterococcus, Enterorhabdus, Flavonifractor, Gemmiger, Guggenheimella, Intestinimonas, Lachnospiraceae incertae sedis, Lactivibrio, Lactobacillus, Macellibacteroides, Marvinbryantia, Olsenella, Paraprevotella, Parasutterella, Phascolarctobacterium, Prevotella, Romboutsia, Ruminococcus, Sporobacter, Sporobacterium, Sutterella, Vampirovibrio, Veillonella and Victivallis) showed higher abundance in normotensive subjects. Moreover, some differences were found in the gut microbiota of patients with isolated systolic or diastolic hypertension compared to healthy subjects. It was found that the numbers of Christensenella and Olsenella were significantly inversely related to diastolic blood pressure, while the numbers of Macellibacteroides and Butyricimonas were significantly inversely related to systolic blood pressure. Moreover, the abundance of Clostridioides XIVa and Paraprevotella was significantly positively associated with diastolic blood pressure [23]. In the study by Li et al., the gut microbiota was analyzed in patients with prehypertension (n = 55), with hypertension not undergoing antihypertensive therapy (n = 99) and in 41 healthy subjects. All study participants were Asian and lived in China. Bacterial DNA was isolated from stool samples, the bacterial 16S rRNA gene was amplified, and its hypervariable regions were analyzed. In patients with prehypertension and hypertension, a decrease in the richness and diversity of the gut microbiota was demonstrated. In patients with prehypertension and hypertension, an increase in the number of Prevotella, Klebsiella, Porphyromonas and Actinomyces and a decrease in the number of Bacteroides, Faecalibacterium, Oscillibacter, Roseburia, Bifidobacterium, Coprococcus and Butyrivibrio were found [24]. The study by Louca et al. analyzed the gut microbiota in 871 women who participated in the Twins UK study (397 women with hypertension and 474 women with normal blood pressure). Bacterial DNA was isolated from stool samples, the bacterial 16S rRNA gene was amplified, and its hypervariable regions were analyzed. In women with hypertension, a decreased diversity of gut microbiota (measured by the number of amplicon sequence variants – ASV) was found. An increase in the number of Erysipelotrichaceae UCG-003 and a decrease in the number of Ruminiclostridium 6 were demonstrated [25]. In a study by Palmu et al., which included 6953 subjects aged 25–74 living in Finland, participants of the FINRISK 2002 study (55% women and 45% men; 47% had hypertension), the composition of the gut microbiota was also analyzed. Bacterial DNA was isolated from stool samples, the bacterial 16S rRNA gene was amplified, and its hypervariable regions were analyzed. There were significantly negative relationships between the Shannon index (a measure of the diversity of gut microbiota) and systolic (β = –0.5; p = 0.01) and diastolic (β = –0.3; p = 0.02) blood pressure. Moreover, significant relationships were found between the number of individual bacteria and the occurrence of hypertension [26]. In the study by Sun et al., which included 529 subjects aged 48–60 years living in the United States, participants of the Coronary Artery Risk Development in Young Adults (CARDIA) study, the composition of the microbiota was also assessed. Bacterial DNA was isolated from stool samples, the bacterial 16S rRNA gene was amplified, and then its hypervariable regions were analyzed. A significant relationship was found between the number of individual bacteria and systolic blood pressure. There was a significant negative relationship between the number of bacterial genes (a measure of microbiota richness) and systolic blood pressure (β = –1.8; p < 0.02). Moreover, there was a significant negative relationship between the Shannon index (a measure of the diversity of intestinal microbiota) and systolic blood pressure (β = –1.7; p < 0.02). It was found that a greater abundance of bacterial species was associated with a lower risk of hypertension [OR = 0.75 (95% CI: 0.60–0.94)] [27]. In the study by Silveira-Nunes et al., the richness and diversity of the gut microbiota of healthy subjects (n = 32) and patients with hypertension (n = 48) were analyzed using 16s rRNA gene sequencing. In patients with hypertension, a decrease in the abundance of the Bacteroidetes phylum was demonstrated (p = 0.03), which contributed to an increase in the Firmicutes/Bacteroidetes ratio. Moreover, in patients with hypertension there was a reduced number of bacteria from the Lachnospiraceae and Ruminococcaceae families, including Roseburia, Coprococcus and Oscillospira, producing butyrate (short-chain fatty acid), and an increased number of Akkermansia and Lactobacillus [28]. In a study by Takagi et al., including 239 Japanese people – healthy people, patients with hypertension, patients with dyslipidemia, and patients with type 2 diabetes – changes in the gut microbiota were assessed depending on the co-occurrence of these diseases. Stool samples were analyzed by 16s rRNA gene sequencing. It was found that in patients with hypertension there was an increased number of Actinobacteria (p < 0.01), Bifidobacterium, Collinsella (p < 0.01) and Escherichia with a simultaneous decrease in the number of Bacteroidetes (p < 0.05) [29]. In a study by Yan et al., which included 60 subjects with normal blood pressure and 60 patients with hypertension, differences in the diversity of gut microbiota were analyzed using metagenomics methods. An association study of the entire metagenome (the pool of DNA of organisms inhabiting a given environment) showed that 53,953 genes of the gut microbiota differed in their distribution between healthy and hypertensive subjects. Opportunistic pathogenic bacteria such as Klebsiella spp., Streptococcus spp. and Parabacteroides merdae were frequently present in the gut microbiota of hypertensive patients, while the abundance of short-chain fatty acid-producing bacteria such as Roseburia spp. and Faecalibacterium prausnitzii was reduced [30]. In a study by Kim et al., including 18 participants with normal blood pressure and 22 patients with hypertension, differences in the diversity of the gut microbiota were analyzed. In patients with hypertension, a decrease in the number of the most important butyric acid-producing bacterium – Eubacterium rectale – was found. Moreover, the number of Parabacteroides johnsonii and Alistipes finegoldii increased, while the number of Bacteroides thetaiotaomicron decreased [31]. In a meta-analysis of 19 studies by Cai et al., including 17,944 participants, it was found that patients with hypertension had a lower Shannon index (a measure of the diversity of the gut microbiota) (SMD = –0.13; 95% CI: –0.22 to –0.04) and a higher ratio of Firmicutes (Gram-positive)/Bacteroidetes (Gram-negative) bacteria (SMD = 0.84; 95% CI: 0.10–1.58) [32].
Overall, patients with hypertension show reduced richness and diversity of the gut microbiota, which is associated with elevated blood pressure. Numerous differences in the numbers of individual bacteria in the gut microbiota have been identified between hypertensive and normotensive individuals [24–27].
The role of gut dysbiosis in the pathogenesis of hypertension
The results of the experiments on rats and mice described below indicate that dysbiosis has a hypertensinogenic effect.
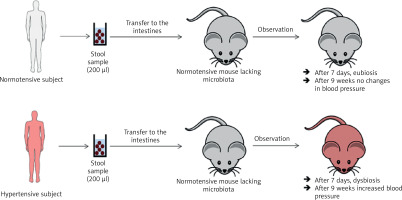
In a study by Toral et al., the gut microbiota was transferred (for three consecutive days and then once every 3 days for 4 weeks) from a spontaneously hypertensive rat (SHR) to a normotensive rat (Wistar Kyoto, WKY). After 4 weeks of observation, dysbiosis and increased blood pressure were observed in the WKY rat (Figure 3) [33]. In the previously cited study by Li et al., the gut microbiota was transferred (twice, one day apart) from a person with normal blood pressure to 5 microbiota-free mice (GF C57BL/6L), and the gut microbiota was transferred from 2 hypertensive patients to 10 microbiota-free mice (GF C57BL/6L) (Figure 4) [24]. In mice that received microbiota from hypertensive patients, dysbiosis was found after 7 days and after 9 weeks blood pressure increased. In hypertensive mice, the abundance of Anaerotruncus, Coprococcus, Ruminococcus, Clostridium, Roseburia, Blautia and Bifidobacterium was found to be decreased, while the abundance of Coprobacillus and Prevotella was increased. In mice that received microbiota from subject with normal blood pressure, eubiosis was observed after 7 days, and after 9 weeks there were no changes in blood pressure [24].
Figure 3
Transfer of intestinal microbiota from a hypertensive rat to a normotensive rat and from a normotensive rat to hypertensive rat. Based on [32]
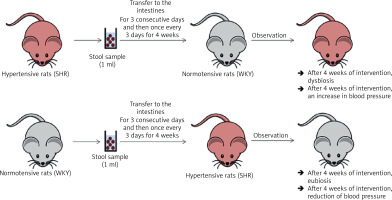
Figure 4
Transfer of intestinal microbiota from a healthy person with hypertension to mice devoid of microbiota. Based on [33]
The human intestinal wall is the surface connecting the body with the external environment. Parallel tight junctions between colonocytes, constituting an intestinal barrier, protect against the penetration of pathogens, toxic substances and pro-inflammatory factors into the bloodstream. Dysbiosis leads to impaired function and increased permeability of the intestinal barrier [34].
Rats with hypertension (SHR) showed morphological features of intestinal damage associated with intestinal barrier disorders (reduced villi length) and signs of inflammation in the large intestine [35]. Another study reported increased permeability of the intestinal barrier and decreased expression of proteins forming tight junctions, the activity of which determines the efficiency of the intestinal barrier, i.e. occludin, Tjp1 protein, and cingulin [36].
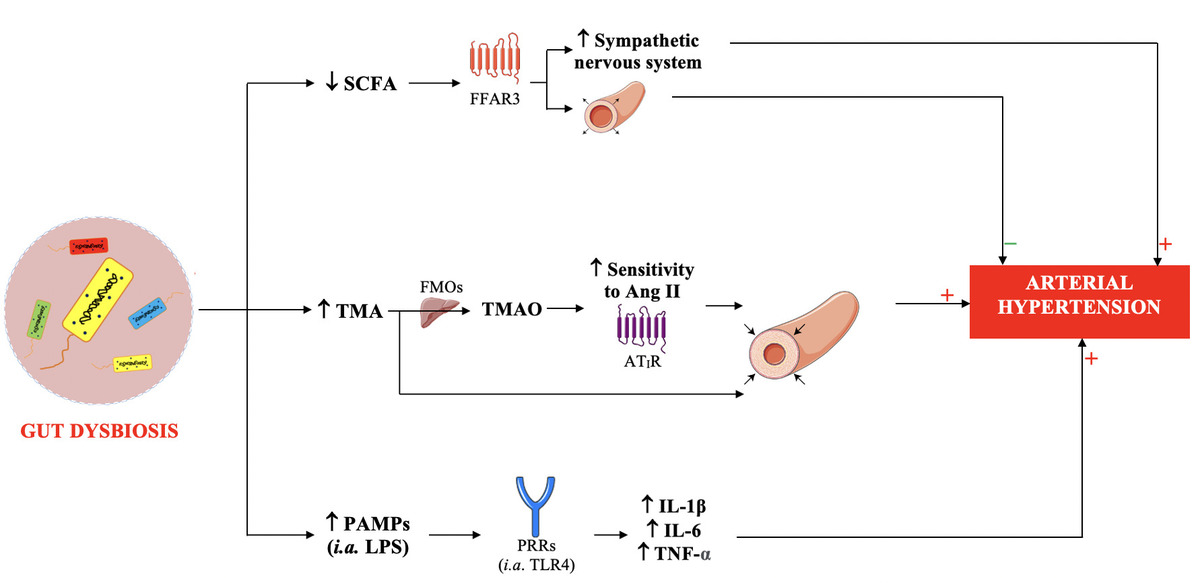
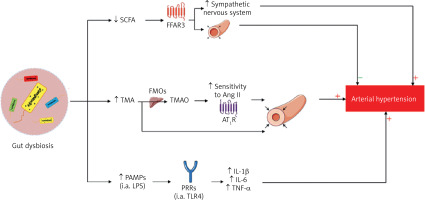
Dysbiosis occurring in patients with hypertension increases the permeability of the intestinal barrier to, among other substances, hypertensinogenic chemicals (trimethylamine, TMA) and pathogen-associated molecular patterns (PAMPs), and is associated with reduced production of antihypertensive compounds (SCFAs) (Figure 5) [37].
Figure 5
Mechanisms of the involvement of intestinal dysbiosis in the pathogenesis of hypertension
SCFA – short-chain fatty acids, TMA – trimethylamine, PAMPs – pathogen-associated molecular patterns, LPS – lipopolysaccharide, FFAR3 – free fatty acid receptor 3, FMOs – flavin-containing monooxygenase, TMAO – trimethylamine N-oxide, Ang II – angiotensin II, AT1R – angiotensin II type 1 receptor, PRRs – pathogen recognition receptors, TLR4 – toll-like receptor 4, IL-1β – interleukin 1β, IL-6 – interleukin 6, TNF-α – tumor necrosis factor α.
Short chain fatty acids
In patients with hypertension, the number of SCFA-producing bacteria is reduced [28, 30].
SCFAs, including acetic, propionic and butyric acids, are produced by anaerobic fermentation of indigestible carbohydrates of plant origin (including fructopolysaccharides, galactopolysaccharides and resistant starch) by gut bacteria including Bacteroidetes. Dysbiosis (in which a decrease in the number of Bacteroidetes is observed) may lead to reduced SCFA production [38]. SCFAs are a source of energy for colonocytes (they ensure the proper functioning of the intestinal barrier and have a local anti-inflammatory effect) and for the microbiota (by stimulating the growth of commensal and symbiotic bacteria, they inhibit the development of other pathogens competing for the place of colonization) [39].
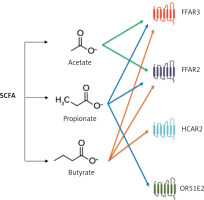
SCFAs that are not metabolized by colonocytes are transferred to the plasma. SCFA-FFAR receptors (mainly FFAR3) (Figure 6) are present, among other locations, on the smooth muscle cells of blood vessels and on the ganglia cells of the sympathetic nervous system [40]. Stimulation of FFAR3 leads to relaxation of the smooth muscles of blood vessels and a reduction in blood pressure [40]. Animal experiments indicate that administration of acetic acid reduces blood pressure in deoxycorticosterone-induced hypertensive mice; propionate acid reduces blood pressure in mice with angiotensin II-induced hypertension, while butyric acid reduces blood pressure in rats [38, 41].
Figure 6
Interaction of short-chain fatty acids with receptors. Based on [39]
FFAR3 – free fatty acid receptor 3, FFAR2 – free fatty acid receptor 2, HCAR2 – hydroxycarboxylic acid receptor 2, OR51E2 – olfactory receptor family 51 subfamily E member 2.
In a randomized clinical trial by Roshanravan et al., including 30 patients with type 2 diabetes, the effect of butyric acid at a dose of 600 mg (6 × 1 tablet/day) versus placebo on blood pressure was assessed. A reduction in systolic and diastolic blood pressure from 135/86 to 129/78 mm Hg (p = 0.013 for diastolic blood pressure) was demonstrated under the influence of butyric acid [42]. In the study by Nakai et al., which included 70 subjects, it was found that in patients with hypertension, FFAR2 receptor expression was significantly lower compared to people with normal blood pressure [43]. The FFAR2 receptor is located mainly on the surface of immune system cells, and its stimulation by SCFAs is associated with anti-inflammatory effects [43].
Trimethylamine N-oxide (TMAO)
Trimethylamine (TMA) is a product of the transformation of carnitine, choline and phosphatidylcholine originating mainly from meat, fish and eggs by bacteria found in the large intestine (including Clostridia, Tenericutes, Proteus, Shigella, Prevotella, and Aerobacter). The number of TMA-producing bacteria is elevated in a state of dysbiosis. These include, among others, Firmicutes, Clostridia, Tenericutes, Proteus, Shigella, Prevotella, and Aerobacter [44, 45]. In the liver, under the influence of flavin-containing monooxygenase (FMOs), TMA is converted to trimethylamine N-oxide. TMA and TMAO can be identified in plasma (the plasma concentration of TMAO is higher than that of TMA) [44, 45].
Animal experiments indicate that the absorption of TMA in the large intestine is elevated in hypertension. Moreover, it has been found that TMA causes vasoconstriction, and TMAO sensitizes blood vessels to the hypertensinogenic effect of angiotensin II (altering the conformation of the AT1 receptor) (Figure 5) [35, 46, 47].
Intestinal barrier permeability
Tight junctions between colonocytes, constituting the intestinal barrier, protect against the penetration of pathogens, toxic substances, and pro-inflammatory factors into the bloodstream [34, 48]. Dysbiosis leads to impairment of the intestinal barrier [34, 48].
In patients with hypertension, increased concentration of zonulin in the plasma is observed, which indicates damage to the tight junctions between colonocytes, which determine the tightness of the intestinal barrier [31].
PAMPs – molecular patterns of pathogens
Lipopolysaccharide (LPS) is one of the PAMPs, i.e. molecular patterns associated with pathogens. LPS is an endotoxin that is a component of the wall of Gram-negative bacteria. After entering the intestinal barrier, LPS binds to toll-like receptor type 4 (TLR4) present on macrophages [34, 49], thereby stimulating the development of systemic inflammation, which is an important hypertensinogenic factor (Figure 5) [34]. A clinical study by Li et al., including 106 patients with hypertension and 251 subjects with normal blood pressure, showed that the former were characterized by increased LPS serum concentration (p = 0.005) [50].
Extracellular vesicles
A relatively new and interesting mechanism that may link the intestinal microbiota with hypertension involves extracellular vesicles (EVs) secreted by various bacteria, including Akkermansia muciniphila (Am) [51, 52]. Akkermansia muciniphila is a Gram-negative bacterium that is normally present in approximately 3–5% of the human gut microbiota and has been reported to have anti-inflammatory properties or effectiveness against metabolic syndrome [51]. Kim et al. examined the effects of Am EVs in a hypertensive rat model and found that they prevent the development of hypertension in spontaneously hypertensive rats (SHR) associated with a T-cell-mediated anti-inflammatory effect [53]. Am-EV treatment increased CD4 and FOXP double-positive Treg cells and reduced CD4 and IL17 double-positive Th17 cells in both peripheral blood mononuclear cells and the spleen of SHRs. Moreover, Am-EVs reduced T cell-related proinflammatory pathways such as IL-1β, IL-6, and STAT3 phosphorylation in the serum or thoracic aorta of SHRs [53]. Thus, Am EVs exhibit an antihypertensive effect through anti-inflammatory activity.
To summarize, several mechanisms related to gut dysbiosis have been identified that are involved in the pathogenesis of hypertension.
Application of gut microbiota modification in treatment of hypertension
Role of high dietary salt in dysbiosis development
Increased dietary salt may contribute to the development of dysbiosis [54]. In an experimental study by Ferguson et al., the effect of a low- or high-salt diet on dysbiosis-associated hypertension in mice was assessed. High sodium intake (≥ 2.3 g/day) was associated with increased relative abundance of several bacterial taxa, including Prevotella, Ruminococcaceae, and Bacteroides [55]. Mice fed a high-salt diet were characterized by increased intestinal inflammation, including the mesenteric arterial arcade and aorta, with a marked increase in the B7 ligand CD86 and formation of isolevuglandin (IsoLG) protein adducts in CD11c+ myeloid cells. Furthermore, adoptive transfer of fecal material from conventionally housed high-salt diet–fed mice to germ-free mice was found to predispose them to increased inflammation and hypertension [55]. Similar observations were provided by a study on rats by Yan et al. In this study, in Wistar rats 8% sodium supplementation led to changes in microbiota composition and increased systolic and diastolic blood pressure. Moreover, gut microbiota transfer from normotensive donor rats in a normal salt diet to high salt Wistar rats led to normalization of blood pressure. In contrast, gut microbiota transfer from high salt-hypertensive donor rats significantly increased blood pressure in normotensive rats. The compositional changes observed at the taxa level included a reduction of 12 bacteria belonging to the phylum Bacteroidetes, 8 from Firmicutes, and 2 from Proteobacteria. This was accompanied by an increase in the Firmicutes to Bacteroidetes ratio (a surrogate marker of gut dysbiosis) [56].
In humans, higher sodium intake was associated with lower microbiota α diversity and shifted microbiota composition [57]. High dietary salt intake leads to a decrease in the number of Bacteroides fragilis and Lactobacillus sp. [57]. It was reported that a high-sodium diet altered the human gut microbiota composition, with a significant reduction in Bacteroides and an increase in Prevotella compared to a low-sodium diet [58]. From a pathophysiological point of view, a high salt content in the diet leads to a decrease in the number of bacteria producing lactate and butyrate (Lactobacillales, Leuconostocaceae, Bacteroides fragilis) while increasing the number of bacteria associated with inflammatory responses. A change in the profile of mediators secreted by the microbiota is observed: a decrease in anti-inflammatory factors – SCFAs, glucagon-like peptide type 1 (GLP-1) and glucagon-like peptide type 2 (GLP-2); a decrease in the production of arachidonic acid; and an increase in the production of glutamate and corticosterone [59]. This leads to an intensification of the inflammatory process (increased concentrations of IL-17, IL-18, INF-γ, TNF-α) [59, 60]. Excessive intake of salt in the diet may contribute to the development of hypertension with a sodium-sensitive phenotype [61]. Excess dietary salt alters the gut microbiome and activates dendritic cells (DCs) to produce reactive oxygen species (ROS) via NADPH oxidase. ROS production leads to IsoLG-adducted protein formation, presentation of co-stimulatory factor CD86, and secretion of pro-inflammatory factors IL-6 and IL-1β. The activated DCs promote T cell activation and stimulate the release of IL-17, TNF-α, and IFN-γ, leading to salt-sensitive hypertension [62].
Another pathophysiological mechanism linking excessive dietary salt with dysbiosis and hypertension is the stimulation of the mineralocorticoid receptor (MR). It has been demonstrated that excessive dietary salt intake leads to a decrease in the number of B. fragilis, which participate in the production of arachidonic acid (AA). The reduction AA levels leads to an increase in the production of corticosterone as well as an increase in the expression of the MR. As a consequence, there is excessive stimulation of the MR and an increase in blood pressure [56].
Antibiotics
In a study by Galla et al., the effect of antibiotic administration (minocycline and vancomycin) on blood pressure in SHR rats was assessed. The use of these antibiotics was associated with a reduction in systolic blood pressure [63]. In an interesting case report by Qi et al., the use of antibiotic therapy (vancomycin, rifampicin and ciprofloxacin) in a 69-year-old patient with hypertension was associated with a significant antihypertensive effect. This effect was so significant that antihypertensive pharmacotherapy was temporarily discontinued in this patient [64]. Minocycline increases the ratio of Firmicutes (Gram-positive) to Bacteroidetes (Gram-negative), thereby influencing the composition of the gut microbiota. In an observational study by Pepine et al., including 26 patients with treatment-resistant hypertension, the effect of minocycline on blood pressure was assessed. Minocycline in 16 out of 26 patients (62%) led to a reduction in daytime blood pressure measured by ABPM (from 135/74 mm Hg to 124/69 mm Hg). Moreover, a reduction in the number of cells associated with inflammation in the blood was found: CD4+ cells containing CD161+IL17+ and CCR6+ITGb7+CD161+IL17+ antigens [65].
To sum up, the use of selected antibiotics (mainly minocycline) changes the composition of the gut microbiota and thus may affect blood pressure.
Transfer of intestinal microbiota
A meta-analysis of 5 experimental studies by Lin et al. showed that the gut microbiota transfer from hypertensive animals significantly increased systolic and diastolic blood pressure in normotensive animals [66].
In an observational study by Zhong et al., including 73 patients with hypertension, the impact of fecal microbiota transfer (FMT) on blood pressure was assessed. The intervention used included FMT from normotensive donors. It was found that the use of FMT was associated with a reduction in systolic blood pressure by 5.1 mm Hg and diastolic blood pressure by 7.7 mm Hg. The antihypertensive effect of FMT was particularly pronounced in patients who were not treated with antihypertensive drugs and in those to whom FMT was administered rectally [67]. A meta-analysis of 5 studies conducted by Zecheng et al., including obese patients, showed that FMT caused a small but significant reduction in systolic (MD = –4.40 mm Hg; 95% CI: –8.28 to –0.52) and diastolic (MD = –2.58 mmHg; 95% CI: –4.57 to –0.60) blood pressure [68].
In summary, FMT seems to exhibit some antihypertensive effects.
Prebiotics
Prebiotics are non-digestible chemical compounds that, when consumed, stimulate the growth and activity of commensal and symbiotic bacteria living in the large intestine, such as Bifidobacterium, Bacteroidetes and Lactobacillus and, as a result, have a positive effect on the host’s health [69, 70]. Prebiotics include fructopolysaccharides such as inulin, which consists of fructose polymers containing between 2 and 60 sugar units. Inulin occurs in 36,000 plants, including chicory root, dandelion root, elecampane root, and Jerusalem artichoke tuber. Other important prebiotics include galactooligosaccharides and polysaccharides from the soluble fiber fraction [42, 70, 71]. Sugars composed of prebiotics are not digested by human digestive enzymes and reach the large intestine unchanged, where they are metabolized by bacteria. Prebiotics stimulate the growth of commensal and symbiotic bacteria, including Bifidobacterium and Lactobacillus, leading to beneficial changes in the microbiota for the host. The use of prebiotics is safe [70, 72, 73].
In a randomized clinical trial by Dehghan et al., including 46 women with type 2 diabetes, the effect of administering inulin (Frutafit IQ) for 2 months at a dose of 10 g/day (i.e. approximately 2–3 tablespoons) versus placebo on blood pressure was assessed. Systolic and diastolic blood pressure decreased from 131/84 mm Hg to 122/78 mm Hg with the administration of inulin (p < 0.001 and p = 0.01, respectively) [74]. However, in a meta-analysis of 5 randomized clinical trials by Faghihimani et al., including 233 subjects, administration of inulin-like sugars had no significant effect on systolic (WMD = –5.83 mm Hg; 95% CI: –12.49 to 0.82) or diastolic (WMD = –2.62 mm Hg; 95% CI: −6.15 to 0.9) blood pressure [75].
In summary, some prebiotics may exert antihypertensive effects.
Probiotics
Probiotics are microorganisms found in the natural, healthy microbiota of the human large intestine. They are obligate or relative anaerobes, and most often include strains of Lactobacillus, Bifidobacterium, Streptococcus and Saccharomyces. The pharmaceutical form of a probiotic must enable the survival of bacteria during intestinal transit (i.e. the action of gastric juice, bile and digestive enzymes). Probiotics are most often available in the form of a lyophilized product. The preparation should contain 109–1010 colony-forming units (CFU) of live bacteria [69]. Single-strain probiotics are used to achieve a specific single clinical effect, while multi-strain probiotics have multiple targets and sites of action [69]. Numerous clinical studies on the effect of probiotics on blood pressure involving only small groups of patients have been already completed.
A meta-analysis of 23 randomized clinical trials conducted by Qi et al., including 2037 people, showed that the use of probiotics was associated with a reduction in systolic (WMD = –3.05 mm Hg; 95% CI: –4.67 to –1.44) and diastolic (WMD = –1.51; 95% CI: –2.38 to –0.65) blood pressure. A subgroup analysis showed that the antihypertensive effect of probiotics was significant only in patients with hypertension or type 2 diabetes [76]. In a meta-analysis of 26 randomized clinical trials, including 1624 subjects, Zhao et al. assessed the effect of probiotic administration for at least 8 weeks on blood pressure measured at home and in outpatient settings. They found that the use of a probiotic was associated with a reduction in systolic blood pressure (home measurements: –2.18 mm Hg; ambulatory measurements: –2.35 mm Hg) and diastolic blood pressure (home measurements: –1.07 mm Hg; ambulatory measurements: –1.61 mm Hg). The antihypertensive effect of probiotics was significant in patients with hypertension or type 2 diabetes [77]. In a review of 14 meta-analyses, including 15,949 subjects, Zarezadeh et al. also found that the use of probiotics reduced systolic (WMD = –1.96 mm Hg; 95% CI: –2.78 to –1.14) and diastolic (WMD = –1.28 mm Hg; 95% CI: –1.76 to –0.79) blood pressure. Moreover, the antihypertensive effect of the probiotic was greater during longer use and when the CFU was ≥ 1010, as well as in patients with hypertension or type 2 diabetes [78]. In a meta-analysis of 9 clinical trials, including 543 subjects, Khalesi et al. found that the administration of a probiotic led to a reduction in systolic (MD = –3.56 mm Hg; 95% CI: –6.46 to –0.66) and diastolic (MD = –2.38 mm Hg; 95% CI: –3.84 to –0.93) blood pressure. A greater antihypertensive effect was associated with the use of multi-strain versus single-strain probiotics (MD = –5.79 mm Hg; 95% CI: –8.66 to –2.93 versus MD = –0.28 mm Hg; 95% CI: –2.95 to 2.39) and when the intervention lasted more than 8 weeks versus less than 8 weeks (MD = –4.90 mm Hg; 95% CI: –8.41 to –1.40 versus MD = –0.93 mm Hg; 95% CI: –3.71 to 1.86) [79]. A meta-analysis of 25,973 participants by Teo et al. showed that probiotics had a greater antihypertensive effect compared to prebiotics and synbiotics (reducing diastolic blood pressure, WMD = –1.34 mm Hg; 95% CI: –2.14 to –0.55) [80].
To sum up, the features of a probiotic that should be taken into account when choosing it in patients with hypertension include: 1) multi-strain probiotic; 2) beneficial effect on the intestinal barrier; and 3) documented antihypertensive effect. The antihypertensive effect should be expected after at least 2 months of using the probiotic.
Probiotics that meet these criteria are characterized by a beneficial effect on the metabolic profile. In vitro studies have shown that the bacterial strains included in the multi-strain probiotic improve the function of the epithelial barrier, reduce immune reactions that may worsen the function of the intestinal barrier (reduce the stimulation of mast cells), have anti-inflammatory effects such as stimulation of the production of anti-inflammatory interleukin (IL) 10, and have the ability to degrade LPS [81].
In summary, certain selected probiotics, mainly multi-strain probiotics, exhibit antihypertensive effects.
Synbiotics
A synbiotic is a combination of a prebiotic and a probiotic [82]. In a meta-analysis of 11 randomized clinical trials, Hadi et al. found that the administration of synbiotic was associated with a reduction in systolic blood pressure (MD = –3.02 mm Hg; 95% CI: –4.84 to –1.21), but not with a change in diastolic blood pressure (MD = –0.57 mm Hg; 95% CI: –1.78 to 0.64). The antihypertensive effect of the synbiotic was most pronounced during long-term use (≥ 12 weeks) and in subjects < 50 years of age [83]. A meta-analysis of 5 randomized clinical trials by Arabi et al. also showed that the administration of a synbiotic reduced systolic blood pressure (WMD = –1.8 mm Hg; 95% CI: –2.8 to –0.7) [84]. In a meta-analysis of 17 randomized clinical trials, Naseri et al. found that the antihypertensive properties of synbiotics are significantly weaker than those of probiotics [85].
In summary, synbiotics have some antihypertensive properties.
Conclusions
Dysbiosis occurs in patients with hypertension. The gut microbiota produces chemical compounds (SCFAs and TMA) that influence the host’s blood pressure. In a state of dysbiosis, TMA production may increase and SCFA production may decrease. In hypertension, under the influence of dysbiosis, intestinal barrier disorders occur, which may lead to increased absorption of liposaccharide (LPS) with hypertensive properties in the large intestine. The results of clinical trials, limited by the small number of participants, indicate that some prebiotics and some probiotics have some antihypertensive effects. An antihypertensive effect can be expected after the use of some multi-strain probiotics for at least 8 weeks.
Future perspectives
Although significant progress has been made in understanding the interplay between gut microbiota and hypertension, several key questions remain unanswered. Future research should focus on elucidating causal relationships through well-designed longitudinal and interventional human studies. While preclinical models have provided compelling evidence for the role of dysbiosis in blood pressure regulation, translating these findings into clinical practice requires more standardized and reproducible clinical trials.
One promising direction is the identification of specific microbial signatures or metabolites, such as SCFAs and TMAO, that may serve as biomarkers or therapeutic targets in hypertensive patients. In future, personalized microbiome-based interventions, including tailored probiotic or synbiotic formulations, could optimize therapeutic outcomes by addressing individual variations in gut microbial composition and function.
Moreover, the integration of multi-omics approaches – including metagenomics, metabolomics, and transcriptomics – may offer deeper insights into host-microbe interactions and help unravel the complex pathways linking the gut microbiota with vascular and renal physiology.
Finally, regulatory frameworks and safety assessments for microbiota-targeted therapies must evolve in parallel with scientific advancements. A multidisciplinary approach combining microbiology, cardiology, and systems biology will be essential to fully harness the therapeutic potential of the gut microbiome in hypertension prevention and treatment.