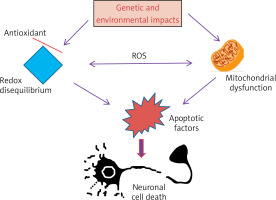
Introduction
One of the most common neurological disorders is Parkinson’s disease. It affects a large number of people, globally estimated as more than 6 million, and it is expected to multiply several times by the year 2040 [1]. The prevalence of the disease is about 1% in individuals aged 60–65 years, increasing to 4% in individuals aged 85 years and over [1]. Parkinson’s disease is characterized by a number of symptoms, the most important of which are some abnormal motor manifestations such as postural instability, tremor at rest, hunched posture, freezing, and slowness of movement. Additionally, there are behavioral and cognitive symptoms, fatigue, autonomic dysregulation, and sleep deprivation [2, 3]. It was found that the disease is associated with a noticeable loss of dopaminergic neurons. It was also found that the remaining neurons contain protein inclusions called Lewy bodies (LBs) [4].
The causative factors leading to the onset of Parkinson’s disease are not exactly known, but it has been found that the majority of patients with this disease have a genetic background (one of the relatives already had this disease). From a genetic point of view, it has been found that patients (5–10%) have individual forms of the gene associated with the onset of the disease and thus have a classic type of Mendelian inheritance. However, there are a number of genetic and environmental risk factors that cause the onset of this disease in most Parkinson’s patients [5].
Increasing age may affect the neurons that produce dopamine in the brain, which in turn may lead to the onset of clinical symptoms of Parkinson’s disease. Thus, the number of dopaminergic neurons in an individual is a major factor influencing the onset of Parkinson’s disease [6]. Furthermore, environmental factors such as exposure to pesticides and accidents including head trauma may play a distinct role in the development of Parkinson’s disease [7]. On the other hand, caffeine consumption and cigarette smoking may reduce the incidence of Parkinson’s disease, although the molecular mechanism remains unclear [8].
Genetic background
Some studies have indicated that there is a genetic mutation associated with the onset of Parkinson’s disease [9]. Although this discovery is from two decades ago, the molecular basis of Parkinson’s disease is still unknown. On the other hand, a genetic study of Parkinson’s disease found several genes associated with its appearance. These genes are LRRK2 (leucine-rich repeat kinase 2), alpha synuclein, parkin, VPS35 (vacuolar protein sorting 35), DJ-1 (protein deglycase) and PINK1 (phosphatase and tensin homologue-induced kinase1). The degree of its appearance depends on whether these genes are in a recessive or dominant form [10]. Also, a study has shown that genetic variants of the methylenetetrahydrofolate reductase (MTHFR) gene are responsible for protecting or causing Parkinson’s disease [10]. For example, genetic variation in the MTHFR gene may be associated with the onset of Parkinson’s disease. However, a polymorphism in a small fragment (C677T) of the MTHFR gene that substitutes the amino acid valine to alanine was associated with reduced susceptibility to Parkinson’s disease among Chinese patients. Therefore, some advanced genetic tools such as next-generation sequencing (NGS), genome-wide association studies (GWAS) and exome sequencing help interpret and understand the molecular basis of this disease [11].
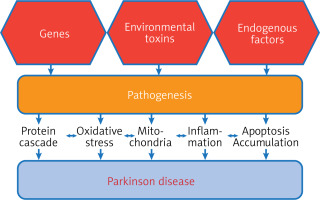
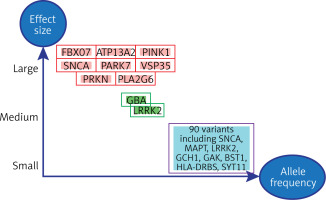
The development of Parkinson’s disease is involved in genetic variation, as 25% of the overall risk of Parkinson’s disease is due to the process of genetic variation [12]. Figure 1 shows the relationship of genetic variation to the severity of Parkinson’s disease, as some genetic variants are involved in the severity of the disease. Certain single genes have enough rare genetic variants to cause the disease. By analyzing the association between families carrying the disease, these single genes which cause Parkinson’s disease were discovered. Among these genes responsible for the onset of Parkinson’s disease are the PRKN, PINK1, and DJ-1 genes. Additionally, mutations in some genes are responsible for the onset of Parkinson’s disease such as mutations in SNCA (α-synuclein gene) and mutations in the PARK7 gene. On the other hand, there are large numbers of common genetic variants that individually contribute a small amount to the risk of Parkinson’s disease. Also in the middle of this spectrum are uncommon (but not rare) variants of intermediate risk, such as variants in the GBA (glucocerebrosidase) and LRRK2 genes [13].
Figure 1
Genetic variants grouped according to their strength of influence on the onset of Parkinson’s disease according to allele frequency [14]
Parkinson’s disease can be stratified according to several common stratification factors which include age of onset (early or late-onset Parkinson’s disease, after reaching age 50), family history, its presence or absence (familial vs. sporadic Parkinson’s disease) and the presence of disease-causing genetic variants (monogenic vs. unknown reason of Parkinson’s disease) [14, 15].
Numerous studies indicated that altered pressures resulting from mitochondrial dysfunction are two important cellular stress factors that accelerate the onset of Parkinson’s disease [16]. Research proved that the performance of neurons and their role perfectly depend on the quality of mitochondria and their vital dynamics, and therefore any change in the activity and function of mitochondria leads to the death of neurons [17]. These conclusions were reached by performing autopsies on brain samples collected from animal models and patients with Parkinson’s disease, where it was discovered that the activity and function of mitochondria were diminished, especially in the first complex activity [18]. In addition, mitochondria help in cellular fragmentation, and therefore it is necessary to constantly examine the dysfunctional organelles [19]. Mitochondria also stimulate the synaptic activity of neurons, especially at the terminal end of the cells, through the optimal distribution of energy to the cellular organelles according to the energy needs of neurons [19].
From a therapeutic point of view, dopamine (DA) is an important substance in the treatment of Parkinson’s disease, and therefore the current treatment strategy for Parkinson’s disease is based on restoring an optimal level of DA and preserving the signaling pathways associated with it. Therefore, in the treatment strategy patients are given the drug levodopa (known as L-DOPA [L-3,4-dihydroxyphenylalanine]), as it is the precursor to dopamine [20]. L-DOPA slows down the progression of Parkinson’s disease. However, the use of L-DOPA in the treatment of Parkinson’s disease is not recommended in the long term because high doses of L-DOPA increase the risk of developing dyskinesia in patients with Parkinson’s disease [21]. Moreover, it was found that giving L-DOPA for a long time increases the side effects represented in problems in the heart, blood vessels and the digestive system. On the other hand, L-DOPA was found to be preferable in combination with carbidopa, a peripheral decarboxylase inhibitor. Therefore when carbidopa is given combined with L-DOPA, the side effects of L-DOPA can be greatly reduced [20].
Recently, there is increasing interest in discovering therapeutic schemes that improve mitochondrial function and improve ROS by using natural compounds that exhibit very low side effects [22]. Certain studies have shown that use of natural plant components collected from the environment have a beneficial effect on Parkinson’s patients [23–25]. Although the exact molecular mechanism of action is not fully understood, one of the most important aims of using these extracts against Parkinson’s disease is to improve ROS production [23]. This review will discuss some herbs that are highly effective in improving the ratio of ROS in cells and thus protecting nerve cells from damage. The most important herbal medicinal plants are: Mucuna pruriens, Bacopa monnieri, Curcuma longa, Withania somnifera, Camellia sinensis and Gingko biloba. The use of such plants will improve the development of treatment strategies for Parkinson’s patients
Etiology
The causes of Parkinson’s disease are still incompletely understood. Genetic and environmental factors are known to play a major role in the occurrence of the disease [26, 27]. Also, advanced age or reaching old age is one of the most important factors associated with onset of Parkinson’s disease, because it is rare for the disease to occur at an early age [28, 29]. The explanation may be that with advancing age and reaching old age, failure occurs in most of the cellular and physiological vital processes, and therefore the dopaminergic neurons do not play their natural role, which exposes them to damage and abnormal pathological effects. The association of neurons with type-L calcium channels is indicative of dopaminergic cell senescence [30].
One of the main reasons for the spread of Parkinson’s disease is the increase in life expectancy, as the increase in industry, which is one of the factors that increase environmental pollution (increased carbon monoxide, organic solvents, and carbon disulfide), is one of the main factors in the emergence of the disease (Figure 2). On the other hand, increased rates of viral and bacterial infections in poor health areas were found to be among the reasons for the increase in Parkinson’s disease [31]. Also, the increased use of pesticides may increase the risk of developing neuronal diseases [32]. Some pesticides such as rotenone and paraquat, which are widely used in agriculture, have shown a significant ability to cause dopaminergic cell death in the substantia nigra (Figure 3) of small laboratory animals [33].
On the other hand, exercise and the use of certain anti-hypertensive and anti-inflammatory drugs (related to the anti-calcium mechanism) reduce the risk of developing Parkinson’s disease, although their role needs further explanation [34, 35].
Pathogenesis
Pathologically, Parkinson’s disease is a group of disabling motor and motor abnormalities, which include tremor, bradykinesia, imbalance, and muscle stiffness. These symptoms may be related or separate to a decrease in the level of dopamine in several parts of the brain, including the putamen, striatum, and caudate nucleus, which results from the deterioration or damage of dopaminergic neurons, which are the main causes of Parkinson’s disease. The disease begins when dopamine levels drop to levels of 80–85% in the striatum and most neurons (50–60% of neurons) are destroyed. There are other factors associated with these manifestations in disease events, such as mitochondrial dysfunction due to increased oxidative stress. Also, what are known as Lewy bodies form as a result of increased levels of proteins in neuronal cytoplasm and increased neuronal loss [36, 37].
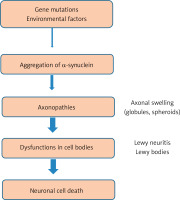
Studies have shown that Lewy bodies are associated with signs of Parkinson’s disease. Where research has proven that Lewy bodies are one of the hallmarks of neurological diseases, when making histological analyzes of α-cell disorders. On the other hand, the formation of Lewy bodies takes place through complex processes, and the role of Lewy bodies in the occurrence of alpha-synucleinopathies needs complex explanations [38]. It was also found that the formation of Lewy bodies in the human brain tissue takes many years [39]. Thus, some studies that showed the difficulty of forming Lewy bodies in laboratory animals is due to the time factor that is needed in order to facilitate obtaining them in histological studies, as the life spans of rodents are shorter than that of humans [40]. This indicates that the occurrence of Parkinson’s disease is easily confirmed when Lewy bodies are present in histological studies (Figure 4).
Recent advancements in understanding the pathophysiology of Parkinson’s disease
There is some evidence that Parkinson’s disease is a syndrome consisting of pathological conditions and not a single clinical entity, and therefore the treatment strategy requires a specific therapeutic approach [41]. In addition, there may be subtypes such as the postural instability and gait difficulty (PIGD) subtype, and the dominant subtype of tremor within Parkinson’s disease [42]. Moreover, the different genetic forms of Parkinson’s disease show distinct and unique phenotypes such as the presence of dystonia and other movement disorders, response to deep brain stimulation (DBS), development of complications of levodopa treatment, and a degree of associated cognitive abilities [43]. Therefore, identifying different phenotypes and understanding the basic pathological mechanisms through genetic analysis helps facilitate access to effective treatment strategies for Parkinson’s disease [44]. This review seeks to contribute to finding experimental treatments using natural compounds which are cheap, safe and available in the surrounding environment [45].
Potential mechanisms of natural products that exert their neuroprotective impacts based on oxidative stress, inflammation, and mitochondrial function
It is known that the neuropathological feature of Parkinson’s disease consists of the accumulation of Lewy bodies and protein aggregates within nerve cells and Lewy neuritis. This occurs as a result of mistreatment of the protein alpha-synuclein and its aggregated forms and also as a result of the gradual loss of nigrostriatal neurons [46]. Recently, natural products have received great attention in the treatment of neurological diseases, including Parkinson’s disease, as a result of their important biological and medical properties [47, 48]. There are three main biological processes involved in the development of neurological diseases, including Parkinson’s disease: inflammatory dysfunction, oxidative stress, and mitochondrial dysfunction (Figure 5) [49]. Studies have shown that oxidative stress is mainly involved in the onset of Parkinson’s disease, Alzheimer’s disease, and other neurological diseases. Oxidative stress plays an important role in catastrophic neurodegeneration as a result of stimulating the increase and attack of free radicals on neurons [50, 51].
Figure 5
Major biological processes involved in neurodegeneration including oxidative stress, inflammatory and mitochondrial dysfunctions [62]
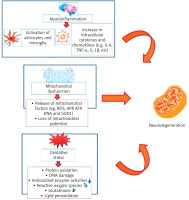
On the other hand, the appearance of oxidative stress is due to a lack of antioxidant defense capacity and the formation of reactive oxygen species (ROS). As a result of mitochondrial dysfunction, cell damage and DNA repair system impairment occur [52]. It was also found that oxidative stress is one of the most important biological processes in the onset of Alzheimer’s disease as a result of stimulating tau protein phosphorylation and exacerbating the generation and aggregation of amyloid β (Aβ) [53, 54].
Another biological process that contributes to the emergence of Parkinson’s disease is neuroinflammation. Pathways of neuroinflammation include the adaptive and innate immune system of the central nervous system, leading to neurodegeneration [55, 56]. It has been found that microglia in the central nervous system are the main component of the innate immune response. In neurological diseases, in response to pathological changes in the nervous system, microglia undergo morphological changes, and these cells secrete a different group of inflammatory mediators, including chemicals, cytokines, and cytotoxic molecules. However, inflammatory mediators function by allowing astrocytes to respond to survival and compensation in response to growth factor and to the process of repairing secondary inflammation [57, 58].
Mitochondrial dysfunction is one of the biological factors that contribute to the emergence of Parkinson’s disease and other neurological diseases. It is known that oxidative phosphorylation occurs in mitochondria and helps maintain a low concentration of Ca2+ ions in the cytosol [59]. However, when the oxidative phosphorylation process is disrupted, it leads to an increase in ROS levels and to excessive absorption of calcium ions, which leads to the opening of mitochondrial pores and a decrease in the function of the mitochondrial membrane [60]. The results have proven that the deficiency of compound I in the substantia nigra of Parkinson’s patients is caused by mitochondrial dysfunction [59, 60]. It was also found that some mitochondrial defects, such as platelets and skeletal muscle, and the appearance of some lymphomas, also play a secondary role in the emergence of Parkinson’s disease [61].
Activity of several natural products against neurodegeneration
Medicinal plant extracts against Parkinson’s disease
Several plant extracts exhibiting effective therapeutic impact against Parkinson’s disease are summarized in Table I.
Table I
Pharmaceutical activities of plant natural products with neuroprotective capacity
| Plant extracts – natural products | Pharmaceutical activities | Animal model | References |
|---|---|---|---|
| Water hyssop (Bacopa monnieri) | Anti-inflammatory Antioxidant Protecting neurons Enhancing memory Strengthening cognitive functions | Animal models Mice Drosophila | [63–67] |
| Velvet bean (Mucuna pruriens) | Anti-nephropathy Anti-inflammatory Anti-ulcer properties Improve neurons | Animal models Humans | [73–75] |
| Indian ginseng (Withania somnifera) | Improve learning and memory Nervous stimulant Antioxidants Antidepressants Anti-cancer Anti-inflammatory Enhancing memory | Animal models Mice | [56] [78–83] |
| Turmeric (Curcuma longa) | Anti-inflammatory Antioxidant Antidepressant Anticancer Improve neurological disorders such as Alzheimer and Parkinson | Mice Rats Human | [87–93] |
| Gingko biloba (Maidenhair tree) | Anti-inflammatory Antioxidant activity Anti-aging Neuroprotective properties | Animal models | [100–105] |
| Camellia sinensis (green tea) | Antioxidant properties Anti-inflammatory Anticancer Anti-arthritic Anti-neuralgic | Mouse model | [108–113] |
| Camellia sinensis (black tea) | Antioxidant capacity Protecting neurons | Rat model | [118, 119] |
| Strawberry | Protection for neurons | Rat model | [123, 124] |
Water hyssop (Bacopa monnieri)
This plant belongs to the creeping perennial herbaceous family and has great medicinal value. These plants possess known medicinal properties such as anti-inflammatory [63], antioxidant [64], neuron protecting [65], antimicrobial [63], and memory enhancing [66] effects. Moreover, it was found that the extract of the water hyssop (WH) plant has great benefit in strengthening cognitive functions [67]. The results of experiments carried out on Parkinson’s disease in animal models exposed to toxins or genetically modified animal models, showed that WH extract given orally for 4 weeks with 15 mg/kg body weight (b.w.) had an anti-Parkinson’s disease effect [68]. It was also found that the Parkinson’s model of mice and Drosophila showed similar results when exposed to rotenone and treated with this extract (5 mg/kg b.w. once daily for 7 days) [69] and it reduced apoptosis and oxidative stress induced by rotenone [70].
According to the previous results, we can conclude that WH plant extract provides clear potential as a natural drug to improve function of the neurons and as an anti-Parkinson’s disease. However, further studies are needed to fully understand the mechanism of action of this extract in order to be used as a possible compound against Parkinson’s disease.
Velvet bean (Mucuna pruriens)
It is a tropical medicinal plant with great pharmacological importance. VB seed has anti-nephropathy, anti-inflammatory and other anti-parasitic and anti-ulcer properties at 350 mg/kg b.w., 530 mg/kg b.w., and 700 mg/kg b.w. orally once daily [71]. The importance of VB roots is due to the fact that it contains levodopa [72], which is considered the gold standard treatment for Parkinson’s disease at 14.4 to 720 mg/day of VB. In addition to levodopa, the seeds of the VB plant contain some other components that act together with levodopa against Parkinson’s disease. Some studies on animal models of Parkinson’s disease [73, 74] and also on humans [75] (0.1% of VB and their equivalent per 100 g fly food, 1.5–2.5 g of VB, respectively) showed that the components of VB seeds improve motor behavior. In addition, the clear biological effect of VB seeds against Parkinson’s disease is shown to be able to reduce oxidative stress [74] as a result of containing mineral chelates and antioxidants [76].
Indian ginseng (Withania somnifera)
This plant is a valued medicinal plant in India, where it is used as a medicine to treat several diseases [77]. Indian ginseng (IG) (10 mg/kg/day ginsenoside Rg3 for 21 day) is used to improve learning and memory, a nervous stimulant, and also a sexual stimulant for men [56, 78]. Several studies have also demonstrated the possibility of using the roots of this plant as antioxidants [79], antidepressants [80], anti-cancer [81], anti-inflammatory [79], and enhancing memory [82]. Also, there are several studies that have proven its effectiveness in improving Parkinson’s disease at 100 mg IG/kg b.w. for 7 or 28 days [83]. IG root extract was also used to reduce oxidative stress resulting from MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) in a mouse model of Parkinson’s disease. This effect is due to the fact that IG (100 mg/kg b.w. orally for 7 days) increases levels of antioxidant enzymes such as glutathione peroxidase (GPx) and glutathione (GSH) and decreases levels of ROS in the neural cells [84, 85]. Moreover, IG extract increases dopamine levels in brain cells and improves motor function metabolism in a mouse model of Parkinson’s disease [83].
Turmeric (Curcuma longa)
This plant is a perennial herb whose roots are frequently used in traditional folk medicine in cases of swelling and sprain resulting from accidents [86]. The medicinal importance of this herb is that it has anti-inflammatory (1–2 turmeric tablets of a standardized turmeric extract daily for 2 months) [87], antioxidant (500 mg turmeric extract daily for 4 months) [88], antimicrobial (175, 129, 219, 217, 163, 293 and 216 µg/ml against several types of bacteria) [89], antidepressant (30 mg/ml/kg b.w. daily for 4 weeks) [90] and anticancer (4 doses of 8 g turmeric) [91] properties. Turmeric also has protective properties for neuronal damage (30 mg/ml/kg) [90], neuronal death, behavioral deficits (30 mg/kg b.w. for 8 weeks) [92], and brain cell damage (1 and 2 mg/kg, i.v.) [93]. It also has great effectiveness against neurological disorders such as Alzheimer’s (10 µmol/l–1 mmol/l) [94] and Parkinson’s disease [95]. One of the most important bioactive compounds in turmeric plant extract is curcumin, which has antioxidant functions and raises dopamine levels in rat models of Parkinson’s disease (up to 1 µM) [95–97]. Furthermore, an advantage of curcumin is that it shows no toxicity (10–400 mg/m2) [98], which provides beneficial opportunities for its use in the treatment of Parkinson’s disease.
Gingko biloba (Maidenhair tree)
Ginkgo biloba (Gb) is a herb that display important medicinal value, especially its seeds, which are widely used in East Asian countries [99]. One of the most important properties of Gb is that it exhibits anti-inflammatory (6 mg/kg for 7 days) [100], antioxidant activity [100], anti-aging and neuroprotective properties (EGb-761 at the doses as indicated every 3 days, up to 2 years old) [101]. It was also found that Gb extract (EGb 761) shows an ability to protect neurons in animal models of Parkinson’s disease [102]. It was also found that EGb 761 extract reduces behavioral deficits and improves locomotor activity and behavioral turnover in a rat model of Parkinson’s disease [103]. Moreover, Gb extract (EGb 761) increases levels and efficiency of antioxidant enzymes such as catalase, SOD and GSH [104] as well as increasing GSH levels. Additionally, Gb extract improved dopamine metabolites and increased its levels, which strengthens its potential role in Parkinson’s treatment. Also, numerous studies have shown that Gb extract (EGb 761) reduces oxidative stress, improves dopamine level, and increases SOD activity in animal models of Parkinson’s disease [105].
Camellia sinensis (green tea)
When Camellia sinensis (Cs) tea leaves are subjected to steaming and then drying, the tea turns into green tea, which has well-known health benefits [106]. The importance of Cs tea is due to the fact that it contains polyphenols [107] that have antioxidant (50 ppm) [108], antimicrobial (0.25–25 µg/ml) [109], anti-inflammatory (50 mg/kg, 100 mg/kg, and 200 mg/kg orally administered) [110], anticancer (400 mg/day up to 1315 mg/day) [111], anti-arthritic (2–12 g/l for 3 weeks) [112] and anti-neuralgic [113] properties.
It was found that Cs tea leaf extract reduces the risk of Parkinson’s disease [114]. Catechins in green tea are the main polyphenol component of this plant. Moreover, there is another important substance in green tea extract called epigallocatechin-3-gallate (EGCG), which showed a great ability to protect neurons in a mouse model of Parkinson’s disease, as it was protective against MPTP-induced onset of Parkinson’s disease. The reason appears that EGCG has metal chelating and antioxidant properties [115]. On the other hand, it was found that the treatment of a rat model (6-OHDA) of Parkinson’s disease with polyphenols improved antioxidation status and inhibited the ROS-NO pathway. Furthermore, polyphenols protect neurons by maintaining increased scavenging free radicals in brain cells [116].
Camellia sinensis (black tea)
Fermentation and oxidation of Cs tea leaves produces black tea, which is widely consumed throughout the world [117]. Black tea contains theaflavin, which has great medicinal value. Theaflavins present in green tea are known for their antioxidant capacity (5–40 µmol/l) [118]. It was also found that black tea extract has the property of protecting neurons (8–10 mg/kg/day; i.p.) [119] in a rat model of Parkinson’s disease (6-OHDA) by protecting DA-ergic neurons in brain cells [120]. So far, the neuroprotective mechanism of theaflavin (polyphenols) found in black tea is not known [118]. Studies have shown that theaflavin is one of the potent types of catechins found in tea leaves [118]. It was found that theaflavin improves brain cells in a rat model of Parkinson’s disease, reducing the risk of MPTP (10 mg; 20 mg; 50 mg/kg b.w.) [121]. It was also found that theaflavin reduces apoptosis by reducing the expression of caspase genes (3, 8, and 9) [121].
Strawberry
It has been shown that strawberry fruit contains many antioxidants such as ellagic acid and caffeic acid, some flavonoids (anthocyanins, tannins, catechins, quercetin, and kaempferol), carotenoids, and vitamins [122]. The mechanism of action of strawberries (1 g/kg orally for 3 weeks) in providing protection for neurons and age-induced disability and improving cognitive function comes through the release of dopamine (DA) and calcium content, and enhancing GTPase activity [123, 124].
Active natural products for Parkinson’s disease treatment
Botanic sources
Baicalein
This compound is extracted from the roots of Scutellaria baicalensis, a plant of the mint family (Labiatae) [125]. The importance of the baicalein compound (5 or 10 mg/kg) is that it prevents the accumulation of ROS molecules, reduces apoptosis, maintains ATP level, and improves mitochondrial membranes in brain cells when exposed to rotenone, which causes neurotoxicity [126]. It has also been found to enhance 5-HTP and dopamine levels (10 mg/kg) [127].
Erythrina velutina extract
This herb is a member of the legume family (Fabaceae) and was found to show a protective effect for nerve cells. When nerve cells were treated with 6-OHDA, which causes neurotoxicity, and then treated with Erythrina velutina compound, a reduction in neurotoxicity and removal of free radicals were observed. The results indicate that Erythrina velutina (0.5 µg/ml) can be used to treat Parkinson’s disease [128].
Resveratrol
This compound is extracted from several plants including berries and grapes. It is chemically a polyphenolic compound [129]. This compound (90 mg/kg RES) has been administered in animal models of Parkinson’s disease and has been shown to prevent oxidative stress, improve neuronal protection and even help with motor deficits [130]. Also, it was found that resveratrol (oral injection of 10, 20 and 40 mg/kg daily for 10 weeks) reduces gene expression of TNF-α and COX-2 genes, prevents chromatin condensation, and maintains mitochondrial vitality [131].
Peganum harmala extract
This plant, commonly called Syrian rue, is a perennial herbaceous plant that typically grows in saline soils near seas such as in the Mediterranean region. When treating animal models of Parkinson’s disease with this extract, it (10 mg/kg) works to reduce neuronal apoptosis, reduce muscle stiffness, and prevent oxidation of proteins and lipids in brain cells [132]. The ability of this extract to reduce the symptoms of Parkinson’s disease comes from its ability to reduce the activity of angiotensin II, which is responsible for the development of symptoms of Parkinson’s disease, and to increase the oxidative stress of neurons [133].
Flavonoids
Flavonoids are compounds that contain polyphenols, a chemical extracted from many natural plants, which have been used in medicine for several years. Among the flavonoids, baicalin, the major metabolite of baicalin (Lamiaceae) is a derivative of Scutellaria baicalensis. This compound (25 and 50 mg/kg) prevents neurotoxicity in animal models of Parkinson’s disease treated with 6-OHDA. This compound shows neuroprotective functions and reduces lipid oxidation [134]. Other flavonoids include apigenin and lutein (1–5 M luteolin for 30 min before lipopolysaccharide treatment), which protect neurons from neurotoxicity such as inflammation and lipid oxidation [135].
Terpenoids
These compounds are extracted from the plant Centella asiatica, which is a medicinal herb found in Asia and some North African countries. These substances are used in the treatment of infections, rheumatoid arthritis, muscular stress and mental insomnia at the concentration of 100 to 200 mg/kg b.w. Among the terpenoids found abundantly in the leaves of this plant, bornyl acetate, α-pinene, β-pinene and terpinene are all monoterpenes [136]. These compounds inhibit activity of the enzyme acetylcholinesterase (AChE). Other plants containing terpenoids, such as Celastrus paniculatus, are used to calm the nervous system as an antidepressant [137]. Derivatives of these compounds are also used in the treatment of tumors in nerve cells of the brain, as it reduces the formation of AB, due to inhibition of amyloid precursor protein (APP). Also, in rats with Parkinson’s disease with memory loss due to exposure to scopolamine, terpenoids facilitated recovery of memory and learning [138].
Phenols
Phenols are chemical compounds found in many plants, the most important of which is the Curcuma longa plant. Curcuma longa extract contains the compound curcumin, which has been used in the treatment of aging since ancient times. Turmeric roots contain curcumin, which has important medicinal properties such as anti-inflammatory and antioxidant [139, 140]. Curcumin (200 mg/day to 6 g/day for up to 8 months) has shown neuroprotective properties by increasing levels of glutathione and antioxidant enzymes and decreasing lipid peroxidation in brain tissue of patients with neurological damage [141, 142]. Besides curcumin, some other compounds found in the Curcuma longa plant also help protect against damage to cells of the nervous system resulting from the formation of β-amyloid, such as D-demethoxycin, demethoxycurcumin, and calpain-A [143].
Alkaloids
Alkaloids are extracted from some plants, such as Hydrangea serrata, which contain the lycopodium alkaloid huperzine A. This compound was tested in animals and cells in vitro and was found to inhibit AChE (0.03 µM (8 µg) and 0.06 µM). (16 µg) [144]. When using this compound at a concentration of IC50 = 0.08 mM, it stopped the AChE enzyme from working completely [33].
Some other alkaloids, such as the alkaloid leonurine extracted from Leonurus heterophyllus (0.2 mg/kg for 7 days), showed a high ability to protect mitochondria and prevent ROS generation and reduce cytochrome c levels in vivo [145]. There are other alkaloids such as berberine, palmatine, coptisine, which are extracted from the roots of the Coptis chinensis plant. These compounds inhibit the action of AChE.
Caffeine
It was found that caffeine in coffee has an effective role against Parkinson’s disease. It was proved that the caffeine found in Coffea arabica, which is widespread in Asian and African countries, shows (at 5 mg/kg i.p. 10 min prior to each MPTP dose) a therapeutic effect in animal models of Parkinson’s disease that were exposed to MPTP [146]. It was found that caffeine improves dopaminergic neurons and improves motor and rotational control in rats with Parkinson’s disease. It was also found that caffeine works to protect dopaminergic neurons and its metabolites in animal models of Parkinson’s disease that were exposed to 6-OHDA [147].
Marine sources
There are many marine organisms that contain natural chemical compounds that have shown pharmacological effects that can be used to treat many diseases [148].
Archaea
Archaea are certain kinds of microorganisms that live in environments with very high temperature or very high salt. These microorganisms contain zwitterionic organic products (up to 1 mM) that help these organisms to withstand high temperatures and protect the particles of these organisms from damage [149]. In addition, such microorganisms which live in hot environments produce mannosylglycerate (MG). MG exhibited significant activity against Parkinson’s disease [150].
Bacteria
Marine organisms provide some compounds with broad biological activity [151]. Among these compounds is NP7, which is produced by Streptomyces bacteria that live in the marine environment. The compound NP7 (0.05 mg/ml) shows antioxidant activity and reduces H2O2 damage. NP7 also protects neurons and reduces apoptosis induced by H2O2. Piloquinones, isolated from Streptomyces sp., have neuroprotective properties [152].
Fungi
Research has shown that some compounds extracted from marine fungi have biological and protective activity against Parkinson’s disease. Among these is neoechinulin A, which is an isoprene quinone alkaloid and is extracted from fungal strains of Aspergillus sp. and Microsporum sp. [153]. Neoechinulin A (0.47 µM) exhibits neuroprotective activity as it preserves mitochondria, reduces lipid peroxidation, and reduces apoptosis [154]. Another substance called cyclonic acid A is extracted from the marine fungi Aspergillus ochraceous and Paecilomyces sp. This substance (at 25 µM) reduces apoptosis as a result of inhibiting expression of apoptosis genes [155].
Algae
Among the important marine algae are microalgae including Haematococcus pluvialis and Chlorella zophingiensis [156]. Seaweed is characterized by its antioxidant chemical compounds such as carotene and astaxanthin [157]. These compounds (6.5 mg/g) have been shown to have a significant ability to prevent and/or delay Parkinson’s disease in rats [158]. Studies have shown that astaxanthin (3 mg/kg/day) activates neurons in the mouse brain [159]. Marine carotene (30, 60, and 90 mg/kg) has also been found to exhibit anti-inflammatory and antioxidant effects [160].
Molluscs
Molluscs are important because they contain staurosporine (AM-2282), an important chemical that inhibits kinase. This substance was first detected in the fungus Streptomyces staurosporeus [161]. Studies have shown that molluscs (at 0.003–0.03 mg/l for 24, 48, and 96 h) of flatworms and sea squirts contain staurosporine (AM-2282) [162], which activates kinase enzymes in primary brain cells and improves their vitality and activity [163]. Also, staurosporine (AM-2282) (3.4 nM) protects neurons from apoptosis and damage [164].
Sea cucumber
Sea cucumber is one of the most important types of marine mollusks, which is characterized by its many vital nutrients [165]. These nutrients activate and treat many neurological diseases, as is known in many countries of the East. Among the important compounds in sea cucumbers, butanol (WBBU) and ethyl acetate (WBEA) were extracted from sea cucumber Holothuria scabra. These substances (at 0.4–1.0 g/kg) have demonstrated a high ability to protect neuronal cells, reduce lipid oxidation, and reduce apoptosis in C. elegans [166].
Also present in the sea cucumber Cucumaria frondosa are some important compounds such as SCG-1, SCG-2 and SCG-3 that prevent Cucumaria frondosa neuronal damage [167]. Thus, these substances extracted from sea cucumbers (at 4 µg/ml [10 µM]) showed a high ability to protect against Parkinson’s disease [167].
Current challenges and limitations in existing Parkinson’s disease therapies
Recently, several studies have been conducted to find novel therapeutic tools to treat or alleviate the devastating symptoms of Parkinson’s disease, whether motor or non-motor [168, 169]. The therapeutic tools mainly depend on identifying receptors, DNA and stem cells [169, 170]. Among the recent technologies in treating Parkinson’s disease is nanotechnology, which has been used with the aim of delivering molecular drugs in small quantities in the brain to reduce the side effects of various drugs and medications [171]. Through these recent treatment technologies, nanomaterials were delivered through the nose directly to the brain, following specific methods of nanomedicine, in order to meet the challenges associated with treating Parkinson’s disease [171]. However, all of these novel methods are still in the experimental stage to determine their effectiveness and degree of safety.
Nanoparticles (NPs), due to their small size and exclusive properties, have become widely utilized in several fields such as nanocarriers for drugs and nanomedicine [172, 173]. Despite these benefits, NPs show some toxicity to human tissues and cells depending on their various properties such as size [174], shape, functional surfaces [170] and concentration [175]. Several studies have shown that the chemicals involved in the manufacture of NPs during their chemical synthesis are responsible for their high toxicity to human cells [176]. It has also been found that biosynthesized nanoparticles show less toxicity when interacting with cells, compared to those prepared chemically [177]. Therefore, one of the main goals of this review is to shed light on the natural compounds that are known to be safe and do not show toxicity to human cells.
Future research aspects in neurodegenerative therapy using natural products
Among the recent technologies applied in treating Parkinson’s disease is nanotechnology, which has been used with the aim of delivering molecular drugs in small quantities in the brain to reduce the side effects of various drugs and medications. The rapid progress in nanotechnology and the ability to control the shape and size of nanoparticles, combined with the large number of medicinal plants, raises the possibility to find a successful treatment strategy for Parkinson’s disease in the near future. Despite this, developing plant nanomedicines to treat neurological diseases is not easy and faces many challenges. Studies have shown that the progress in clinical results lags far behind the success in pre-clinical results in the extent of response to plant nanomedicines [178]. Among the reasons for the weak response in clinical results are the length of time, cost, and complexity of nanomedicine technology compared to traditional formulation technology, which relies on easy treatment in the form of appropriate doses (whether capsules, tablets, or injections) [179].
Resolutions must be found to the main issues associated with the clinical development of nanomedicines compared to current treatments, including biocompatibility and safety, large-scale manufacturing, and overall cost effectiveness [180]. There is hope that these major issues can be overcome through careful planning to reduce the complexity of nanomedicine design, and better control over the chemical and physical properties of nanomedicine (e.g., size, shape, and density of nanomaterials). Also, non-toxic, biocompatible and biodegradable compounds must be used to produce the nanoplatform in clinical trials [179, 180].
Discussion
The causes of Parkinson’s disease are still not completely clear. On the other hand, a lot of research has been conducted, which proved that the most important reasons for the emergence of this disease are the occurrence of a clear defect in the vital functions of the mitochondria and the instability of the redox process [181]. To date, there is no drug available that has the ability to stop or slow Parkinson’s disease without side effects. As a result, the search for a new drug and the development of a treatment strategy are among the main goals in the treatment of Parkinson’s disease. Therefore, this review article aims to discuss the role of a number of natural herbs that have been investigated in many studies, and which have proven to be useful in treating or preventing Parkinson’s disease.
A number of natural extracts and active substances from plants and marine organisms were discussed in terms of their ability to protect neurons and reduce toxicity resulting from drugs that were used to produce animal models of Parkinson’s disease. The safety of these extracts and active substances as well as their lack of side effects when ingested was also discussed. This review article discusses a few of these extracts and natural active substances, depending on their safety and low toxicity, as well as their effectiveness in protecting mitochondria and reducing oxidative stress and inflammation. Any defect in the mitochondrial function and increased oxidative stress may lead directly to the events of Parkinson’s disease (Figure 6). Each plant extract was discussed separately and the extent to which it contains effective substances in protecting nerve cells and brain tissues. Also, the ability of each extract or natural materials was discussed in terms of the availability of benefits, whether in animal models or in patients as a result of their genetic background to Parkinson’s disease or those who were injured as a result of exposure to toxic chemicals such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), rotenone, PQ (paraquat) and other toxic substances that cause Parkinson’s disease. It has been proven that most of these effective natural extracts or substances provide better efficacy and protection when compared to using levodopa.
In recent years, interest in phytochemicals has increased because they have shown high safety for patients in clinical studies compared to synthetic chemicals that have shown high toxicity and a potential to cause some serious health disorders [182, 183]. Polyphenols are among the antioxidant phytochemicals that have been successfully used in nutritional or oral therapies. Polyphenols have become excellent options for their ability to fight free radicals that cause oxidative damage to nerve cells [184, 185]. While the clinical trials that used phytochemicals did not prove any side effects of these materials, such research is still in its initial stages compared to the clinical trials that used synthetic chemicals. Many phytochemicals are characterized by their ability to cross the blood-brain barrier and enter brain tissues with relative ease due to their chemical properties, such as being naturally lipophilic [186]. Moreover, phytochemicals offer faster metabolism, enhanced bioavailability, and better affinity for receptors. Therefore, it makes it easier for the patient to speed up the process of nerve cell function and reduce the effects of the disease symptoms if it is taken regularly and consistently [187–189]. As a result of the importance of these natural plant materials in treating Parkinson’s disease and other neurological diseases, more research must be conducted in real life to investigate the effectiveness of plant extracts and active ingredients in treating such diseases. Increasing the intensity of conducting such experiments using natural materials will greatly help in gaining a more in-depth understanding and interpretation of the components of natural extracts, their mechanism of action, and their effect on nerve cells.
Conclusions
This review provides useful information for the use of natural substances regarding toxicity, potency, or pharmacological significance for therapy of Parkinson’s disease. It was observed that most of these natural substances that were selected and discussed in this review work to protect the dopaminergic neurons in the substantia nigra region of the brain, as they activate the mitochondrial function in these cells. The present report demonstrated the therapeutic ability of natural compounds to improve mitochondrial function and reduce the generation of reactive oxygen species, and the very limited side effects of these compounds. Therefore, many studies have proved that the use of natural plant components has a beneficial effect on Parkinson’s patients. Even though the exact molecular mechanism of action is not fully understood, one of the most important goals of using these extracts against Parkinson’s disease is to improve mitochondrial function in producing reactive oxygen species and thus protecting neurons from damage. Although this review provides important information for Parkinson’s disease and the possibility of providing natural materials to reduce the damage that occurs in nerve cells, the following points are still needed: (1) the chemical components of each plant or animal extract, (2) the mechanism of action of each extract, in terms of molecular and biological pathways associated with it, (3) information on the effectiveness and safety of each compound of the extracts applied against Parkinson’s disease, (4) details on therapeutic results for each compound in patients by conducting many clinical studies and not being satisfied with the results obtained from animal models (in vivo) or cell lines (in vitro) for Parkinson’s disease. All this information will help pharmacologists and physicians to develop new strategies in the development of Parkinson’s disease treatment.