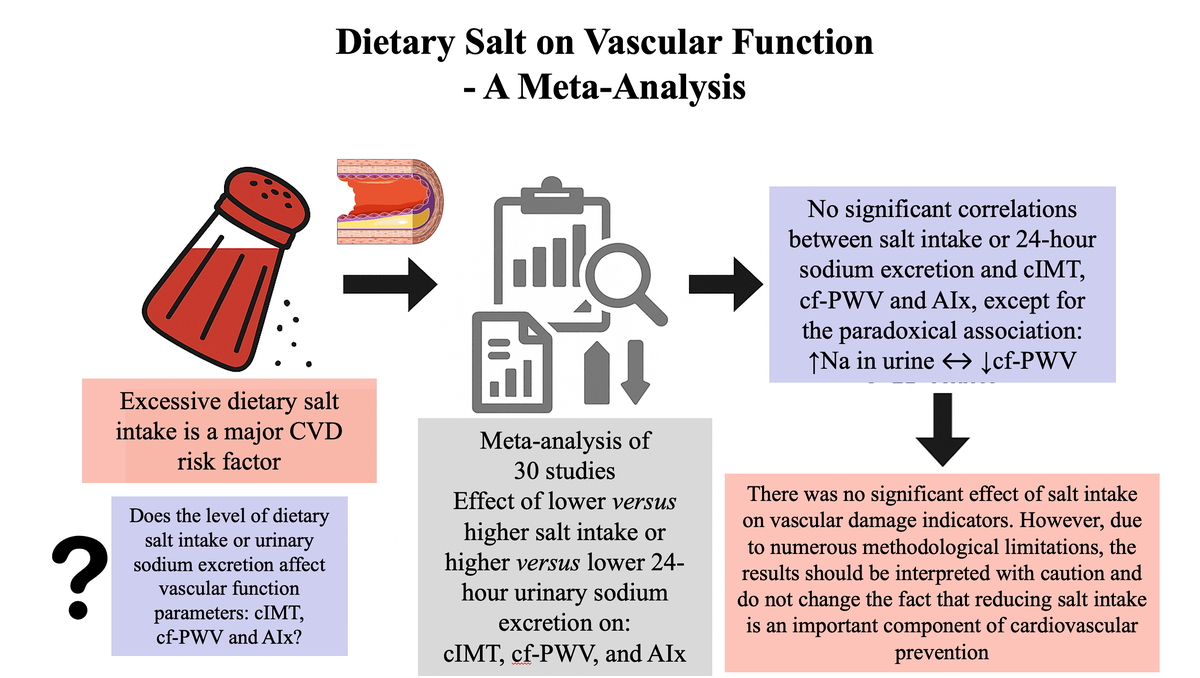
Excessive dietary salt intake is a recognized atherosclerotic cardiovascular disease (ASCVD) risk factor [1, 2]. Research results indicate that excessive dietary salt intake accelerates the progression of atherosclerosis independently of the effect on blood pressure [1, 3].
Current guidelines indicate that acceptable salt intake is < 5 g (or 1 tsp)/day [4]. The average daily salt intake worldwide is twice the accepted level (10.8 g/day). Most countries are characterized by moderate-high salt intake (5.75–11.5 g and > 11.5 g of salt/day, respectively) [5].
In 2019, a total of 18.6 million people died due to cardiovascular disease (CVD), of which dietary risk accounted for 6.9 million, while excessive salt in the diet for 1.72 million [6]. Considering not only CVD, excessive salt consumption is associated with 3 million deaths per year [7].
Excessive dietary salt intake accelerates the progression of atherosclerosis through indirect mechanisms (increased blood pressure, exacerbation of metabolic disorders) and direct mechanisms (induction of oxidative stress, inflammation and damage to the glycocalyx of vascular endothelial cells, impaired immune system function) [1].
The evidence supporting global actions for a moderate reduction in salt consumption to prevent cardiovascular disease is strong [7]. In the United States, a 3-g/day reduction in salt intake could prevent ≈146.000 new cardiovascular disease cases and > 40.000 deaths per year [7]. It is worth mentioning that, for example, reducing the amount of salt consumed in Poland by 30% or to a maximum of 5 g/day could translate into a reduction in the prevalence of stroke and ischemic heart disease by 13.5% and 23.1%, and 8.9% and 15.5%, respectively [8].
Taking into account the above-mentioned relationships, we conducted a meta-analysis in which we assessed the influence of dietary salt intake or urinary sodium excretion on vascular function parameters: carotid intima-media thickness (cIMT), carotid-femoral pulse wave velocity (cf-PWV) and augmentation index (AIx), which are recognized markers for assessing the severity of atherosclerosis [9].
Methods
A systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. We conducted a comprehensive search of PubMed, Scopus, and Web of Science from their creation until January 2025. The search approach incorporated a blend of keywords and MeSH terms: “salt intake”, “sodium excretion”, “carotid intima-media thickness”, “pulse wave velocity”, “augmentation index”, “cIMT”, “cf-PWV”, and “AIx”. Furthermore, we conducted a thorough review of the reference lists of chosen papers to uncover additional pertinent studies. This meta-analysis included adult human participants (P) in whom dietary salt intake, assessed by dietary questionnaires or 24-hour urinary sodium excretion (I) was compared with lower sodium intake or different sodium exposure levels (C) to evaluate its effect on carotid intima-media thickness, carotid-femoral pulse wave velocity, and augmentation index (O) using randomized controlled trials, cohort studies, or cross-sectional designs (S).
Statistical analysis
We performed statistical analyses using Review Manager (RevMan) version 5.4 and Stata version 16.0. Continuous outcomes/variables were pooled using mean differences (MD) with 95% CI. Random-effects models (DerSimonian–Laird) were applied to account for between-study heterogeneity. A flowchart of the participant selection and screening process, and characteristics of the included studies are presented in the supplementary material (Supplementary Table SI, Supplementary Figure S1).
Results
The meta-analysis of cIMT included 2 studies that analyzed low and high salt intake (n = 2013 participants) and 5 studies that compared urinary sodium excretion (n = 1984 participants) and showed no significant association between the assessed parameters (MD = –2.24; 95% CI: –6.66, 2.18 and MD = –0.00; 95% CI: –0.02, 0.02, respectively) (Figure 1 A).
Figure 1
A – The influence of salt intake (low versus high) and urinary sodium excretion (low versus high) on carotid intima-media thickness (cIMT). References in appendix. B – The influence of salt intake (low versus high) and urinary sodium excretion (low versus high) on carotid-femoral pulse wave velocity (cf-PWV). References in appendix. C – The influence of salt intake (low versus high) and urinary sodium excretion (low versus high) on augmentation index (AIx). References in appendix

Similarly, for cf-PWV, no significant effect of dietary salt was demonstrated either as assessed by comparison of low and high dietary salt intake (17 studies with 8276 participants, MD = –0.12; 95% CI: –0.28, 0.04). Higher urinary sodium excretion was associated with better cf-PWV (4 studies with 3109 participants, MD = 1.01; 95% CI: 0.28, 1.74) (Figure 1 B).
Furthermore, no significant effect of dietary salt on AIx was demonstrated either as assessed by salt intake (13 studies with 2078 participants, MD = –1.84; 95% CI: –4.95, 1.27) or urinary sodium excretion (2 studies with 1257 participants, MD = –1.20; 95% CI: –6.38, 3.98) (Figure 1 C).
Discussion
The present meta-analysis aimed to evaluate the impact of dietary salt intake – quantified either as self-reported dietary sodium consumption or 24-hour urinary sodium excretion – on surrogate markers of vascular structure and function (cIMT, cf-PWV and AIx). Despite robust epidemiological evidence linking high sodium intake to elevated blood pressure and increased cardiovascular risk, our meta-analysis did not identify significant associations between salt intake and these vascular parameters, with the exception of an unexpected positive association between urinary sodium excretion and cf-PWV in a limited number of studies.
Several pathophysiological pathways support the hypothesis that high sodium intake may adversely affect vascular health. Excess sodium can elevate blood pressure through increased plasma volume and systemic vascular resistance, accelerating arterial wall remodeling and stiffening [1, 3, 7]. Furthermore, sodium excess has been implicated in endothelial dysfunction via oxidative stress induction, promotion of inflammatory responses, and disruption of the endothelial glycocalyx [1]. Observational studies, including data from the GBD 2017 Diet Collaborators, have suggested that high sodium intake is associated with increased cardiovascular morbidity and mortality worldwide [10].
However, the absence of consistent effects in this meta-analysis aligns with findings from some prior interventional studies where moderate salt reduction over short durations yielded minimal changes in large-artery stiffness indices [11]. The vascular parameters analyzed – cIMT, cf-PWV, and Aix – may require longer exposure times or more extreme sodium intake differences before measurable structural changes occur. For instance, cIMT progression is typically slow, occurring over years, making it less sensitive to dietary interventions of short or moderate duration [12].
The unexpected association between higher urinary sodium excretion and more favorable cf-PWV values in some studies warrants cautious interpretation. This finding may reflect methodological artifacts or be confounding. In particular, a single 24-hour urine collection is subject to considerable day-to-day variability and may misclassify habitual intake [13]. Additionally, reverse causality could be present – individuals with advanced vascular stiffness may have been advised to reduce salt intake, artificially producing a J- or U-shaped association in cross-sectional analyses [14].
There are a number of factors limiting the results of our meta-analysis, including: 1) measurement error – dietary questionnaires tend to underestimate sodium intake, while single-occasion urinary sodium excretion measurements are prone to random error and may not accurately reflect chronic exposure; 2) study design limitations – the majority of included studies were observational, limiting causal inference and leaving results susceptible to residual confounding from unmeasured variables such as potassium intake, overall diet quality, or physical activity; 3) heterogeneity in populations and interventions – differences in baseline blood pressure, antihypertensive medication use, and comorbidities across studies could dilute detectable effects; 4) temporal considerations – arterial structural and functional changes may require prolonged exposure to high sodium before becoming apparent, suggesting that cross-sectional or short-term studies might underestimate the true effect.
From a public health perspective, the absence of strong associations in this analysis should not be interpreted as evidence against sodium reduction strategies. There is overwhelming high-quality evidence that reducing sodium intake lowers blood pressure – a major determinant of cardiovascular events – across diverse populations [7, 15]. Even modest reductions in daily sodium intake have been estimated to prevent tens of thousands of cardiovascular deaths annually in high-income countries [7]. The null vascular findings reported here may simply reflect the insensitivity of the selected endpoints to short-term sodium changes, rather than a lack of harm from high sodium diets.
Further investigation is needed to clarify the sodium–vascular function relationship. Specifically: 1) longer-term randomized controlled trials with precise sodium intake quantification (multiple 24-hour urine collections) are necessary to capture slow-developing vascular changes; 2) inclusion of high-risk populations (e.g., older adults, hypertensive patients) may reveal greater susceptibility to sodium-induced vascular remodeling; 3) advanced vascular imaging (e.g., MRI-based aortic stiffness assessment) may detect subtle early changes missed by traditional cf-PWV and AIx measurements and 4) concurrent assessment of potassium intake is important given its known antagonistic effect on sodium-induced hypertension and vascular damage [5].
In conclusion, based on the results of this meta-analysis, no significant associations were found between dietary salt intake – assessed through either dietary reporting or 24-hour urinary sodium excretion – and investigated vascular parameters, including cIMT, cf-PWV, and Aix. These findings suggest that the direct effects of sodium on the large artery structure and function may be less pronounced or require longer exposure periods to manifest compared with its well-established impact on blood pressure. Further research into sensitive biomarkers of endothelial injury and inflammation may also help elucidate the potential direct effects of dietary salt on atherosclerosis. Clinically, this underscores the importance of maintaining sodium reduction strategies primarily to control hypertension and prevent downstream cardiovascular events, rather than expecting immediate improvements in vascular stiffness indices. Given the methodological limitations and heterogeneity of the available evidence, long-term, rigorously designed trials with accurate sodium assessment remain essential to fully elucidate the vascular consequences of chronic high sodium intake.