The advent of highly effective glucagon-like peptide-1 receptor agonists (GLP-1 RAs) offers transformative potential for obesity management [1–3]. While US studies have highlighted vast eligibility [4] and noted low but increasing initiation rates of anti-obesity medications (1.8%) with significant access disparities [5, 6], the situation in healthcare systems with substantial out-of-pocket costs warrants specific examination. In Poland, GLP-1 RAs are approved for adults with obesity (body mass index (BMI) ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2 with at least one weight-related comorbidity) but remain unreimbursed by the National Health Fund for weight management indications.
This study aimed to quantify temporal trends in GLP-1 RA initiation rates specifically for weight management among eligible adults without diabetes within a large Polish private healthcare network, examining uptake patterns and estimating the national burden of the unmet need in this challenging financial landscape.
Methods
We conducted a descriptive, retrospective analysis of electronic medical records from LUX MED [7, 8], a major private healthcare provider in Poland, spanning January 1, 2018, to December 31, 2024. We included all new adult patients (≥ 18 years) with a recorded BMI ≥ 27 kg/m2 and no diagnosis of type 1 or type 2 diabetes mellitus. Eligibility for GLP-1 RA therapy for weight management was defined as: (1) BMI ≥ 30 kg/m2, or (2) BMI 27–29.9 kg/m2 with at least one documented weight-related comorbidity (arterial hypertension, coronary artery disease, hypercholesterolemia, or sleep apnea). We extracted baseline demographic and clinical characteristics. Analyses were restricted to ‘new patients’, defined as individuals with their first recorded Luxmed encounter during the study period. This criterion ensured consistent capture of baseline BMI, comorbidity, and prescription data from the start of each patient’s record and reduced misclassification of prior GLP-1 RA exposure. The primary outcome was GLP-1 RA initiation (semaglutide (oral or subcutaneous) or tirzepatide) following eligibility determination. GLP-1 RA initiation was defined as the first-ever recorded prescription of any GLP-1 RA agent in the Luxmed database during the study period. Each patient was counted only once, at the date of their first prescription; subsequent prescriptions were not considered additional initiations. Annual initiation prevalence was calculated for each eligible BMI subgroup.
Statistical analysis
Categorical variables were presented as counts (percentages), while continuous variables, expected to follow non-normal distributions, were summarized as medians with interquartile ranges (IQR). We examined temporal trends in annual initiation prevalence. Differences between BMI categories and between sexes were assessed using the Pearson χ2 test. National BMI prevalence data were obtained from Polish epidemiological studies to facilitate extrapolation (Supplementary Methods in Supplementary Materials) [9–12]. A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were performed using Statistica version 13.3 (TIBCO Software Inc., USA).
Results
A total of 438,600 unique adult patients without diabetes mellitus (male 53%, median age 44.0 years [IQR 25.0–55.0]) met BMI eligibility criteria (Supplementary Table SI). Of these, 223,773 (51.0%) had BMI ≥ 30 kg/m2, and 214,827 (49.0%) had BMI 27–29.9 kg/m2 (99,135 with ≥ 1 comorbidity; 115,692 without documented comorbidities).
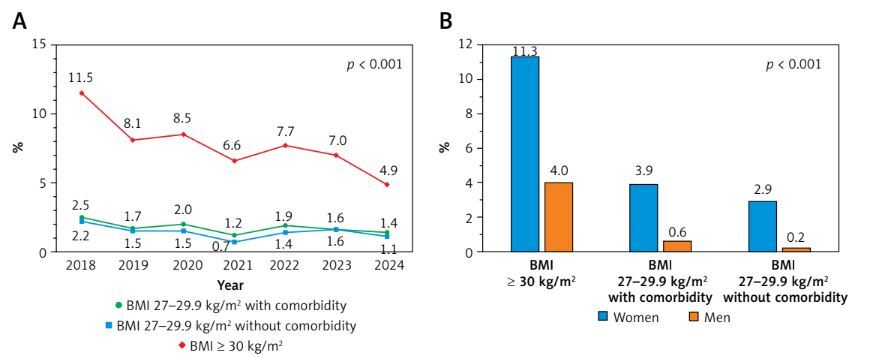
Overall, 20,671 patients (4.7% of the eligible cohort) received a GLP-1 RA initial prescription during the study period (semaglutide (oral or subcutaneous): 77.9%; tirzepatide: 22.1%). Initiation rates were highest among patients with BMI ≥ 30 kg/m2 (7.7%), followed by those with BMI 27–29.9 kg/m2 with ≥ 1 comorbidity (1.8%), and BMI 27–29.9 kg/m2 without documented comorbidities (1.4%). Figure 1 A illustrates the annual GLP-1 RA initiation prevalence from January 2018 to December 2024, stratified by BMI category. Despite expectations of increasing uptake with expanded drug availability, annual initiation prevalence remained persistently low and largely constant, with some groups demonstrating slight declines in recent years.
Figure 1
Glucagon-like peptide-1 receptor agonist (GLP-1 RA) initiation patterns: 6-year trends (A) and sex differences across body mass index (BMI) and comorbidity categories (B)
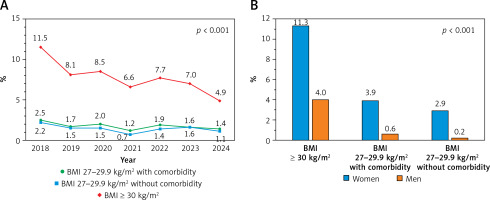
Women were significantly more likely to receive GLP-1 RAs across all BMI categories (p < 0.001 for all comparisons). Among individuals with BMI ≥ 30 kg/m2, 11.3% of women received GLP-1 RAs compared with 4.0% of men. In the BMI 27–29.9 kg/m2 group with at least one weight-related comorbidity, the corresponding rates were 3.9% for women and 0.6% for men. Even among those with BMI 27–29.9 kg/m2 without documented comorbidities, women were more frequently treated (2.9%) than men (0.2%) – Figure 1 B.
Extrapolating our cohort’s GLP-1 RA initiation rate (4.7% cumulative uptake) to national Polish population estimates (approximately 31 million adults, with 8.7–9.3 million estimated as patients without diabetes and eligible for weight management indications based on BMI and comorbidity criteria), we estimate that only 530,000-535,000 individuals – approximately 6% of the eligible population of patients without diabetes – are currently receiving these treatments for weight management in Poland (Supplementary Figure S1).
Discussion
This study underscores a critical and persistent challenge in obesity management in Poland: despite the availability of effective GLP-1 RA therapies, their initiation for weight management in patients without diabetes remains exceptionally low and has shown no sustained growth in recent years within this large private healthcare cohort. The cumulative uptake over approximately 6 years was only 4.7%, with annual initiation prevalence for key eligible groups remaining largely constant or declining, particularly among those with BMI ≥ 30 kg/m2. This starkly contrasts with the sharply rising uptake trends reported in other countries, such as the United States, even where overall initiation rates also remain relatively low [4–6].
The primary barrier is almost certainly the lack of reimbursement for GLP-1 RAs for obesity without concomitant type 2 diabetes in Poland, necessitating full out-of-pocket expenditure. Our data suggest that even among patients using private healthcare – who likely represent a higher socioeconomic stratum/have a higher socioeconomic status – the cost barrier is sufficiently profound to prevent widespread or increasing adoption for weight management. The fluctuating and recently declining annual prevalence in the BMI ≥ 30 kg/m2 group (from 11.5% in 2018 to 4.9% in 2024) is particularly concerning and may reflect initial enthusiasm tempered by cost, medication shortages affecting availability, or saturation of the small population segment able and willing to pay for long-term therapy. This finding is particularly significant given recent evidence from the SELECT trial, where semaglutide (2.4 mg weekly) reduced the risk of major adverse cardiovascular events by 20% in patients with preexisting cardiovascular disease and overweight or obesity but without diabetes [13]. The SELECT trial provides robust evidence that GLP-1 RA benefits extend beyond glycemic control and weight loss to meaningful cardiovascular risk reduction in patients without diabetes highly relevant to our study cohort [1–3, 13]. Indeed, as summarized by Tirandi et al. [3], GLP-1 RAs exert numerous pleiotropic cardiovascular effects, including anti-inflammatory actions, enhancement of atherosclerotic plaque stability, and improvements in cardiomyocyte survival and myocardial contractility, independent of weight loss effects. These established multifaceted benefits make the observed low, stagnant/constant initiation rates for weight management in patients without diabetes in Poland – primarily attributable to financial barriers – an even more critical missed public health opportunity. When extrapolated to the estimated 8.7–9.3 million Polish adults without diabetes eligible for on-label GLP-1 RA therapy, our findings indicate that fewer than 6% have initiated these medications for weight management through private healthcare channels. This problem is further compounded by poor long-term persistence with these agents in both the United States and Poland, even among diabetes patients with reimbursement, where only 6.6% continue therapy for 1 year or more [14–16]. Our recent analysis confirmed that one-third of patients discontinued treatment after a single prescription [16]. This results in high attrition along the treatment pathway: few patients initiate therapy with proven cardiovascular benefits, and even fewer persist, limiting the population-level impact.
The persistent sex disparity in our cohort – where women without diabetes were 3–4 times more likely than men to initiate GLP-1 RA therapy – warrants further investigation. This finding aligns with other reports on anti-obesity medication use and recent data showing greater GLP-1-based therapeutic use among young women compared with men [17]. Several behavioral and system-level factors may contribute. Women more frequently engage with preventive and weight-management services, potentially increasing prescribing opportunities. Societal pressures regarding body weight may heighten the demand among female patients, and physicians may be more likely to prescribe weight-loss pharmacotherapy to women. Structural barriers, such as absent reimbursement for obesity indications in Poland, may differentially impact men and women based on willingness or ability to pay. Biological mechanisms have been proposed, though our study was not designed to test them. Prior reviews note inconsistent sex differences in GLP-1 analog therapeutic effects, though some data suggest women may experience greater weight-loss responses [18] and that GLP-1 receptor activation may more potently suppress food-motivated behavior in females in preclinical models [17]. Other studies suggest women may have higher drug exposure at equivalent weight-adjusted doses or experience more gastrointestinal side effects [17, 19–22]. While such biological differences could influence uptake or persistence, these hypotheses remain speculative given our utilization data. Finally, the ongoing – albeit low – use of GLP-1 RAs among overweight individuals without documented comorbidities likely reflects patient demand or physician discretion beyond on-label criteria. Combined with our prior observation that reimbursement policy changes for type 2 diabetes influence prescribing patterns [7], these findings underscore the unmet need for accessible weight management pharmacotherapy.
Several limitations merit consideration. First, data were sourced from Luxmed, a large private healthcare provider (> 2.5 million patients) in Poland. While this provides a substantial sample, the patient population may not fully represent the entire Polish population, potentially reflecting individuals with higher socioeconomic status, greater medication affordability, different health literacy levels, or better geographic access to private clinics. Detailed data on these factors were not collected. On the other hand, this selection bias should theoretically support higher initiation rates and persistence. An exceptionally low initiation rate was observed even in this potentially advantaged population suggests GLP-1 RA initiation rates in the broader Polish population may be equally concerning, if not worse. Second, our comorbidity ascertainment relied on recorded diagnoses within the Luxmed system, which may be incomplete and could lead to under-documentation of risk factors, potentially misclassifying some patients in the “BMI 27–29.9 kg/m2 without documented comorbidities” group. Third, this observational study cannot determine specific reasons for non-initiation of GLP-1 RAs. Particularly, we lacked data on patients who were offered but declined GLP-1 RA therapy for any reason, including financial constraints. This limitation prevents us from differentiating between low initiation rates due to physician practices versus patient-related barriers such as cost, which is particularly relevant given the high out-of-pocket expenses for these medications in Poland. Fourth, while we focused on initiation, we did not assess medication adherence or persistence, which represent critical additional barriers to effective long-term weight management. Fifth, cardiovascular outcome data were not collected. Sixth, unadjusted comparisons may be subject to residual confounding, particularly for the observed sex differences. Finally, extrapolation to the Polish population involves several assumptions and inherent limitations. The national BMI prevalence data represent the general adult population; while we focused in our cohort and uptake calculations on individuals without diabetes, precisely isolating the non-diabetic segment within each national BMI category requires assumptions, as granular national co-prevalence data for BMI, diabetes status, and specific comorbidities are unavailable. This may lead to some overestimation of the eligible population of patients without diabetes, though the overweight/obese population of patients without diabetes remains substantial regardless.
In conclusion, the promise of GLP-1 RAs for weight management in patients without diabetes in Poland remains largely unrealized. Urgent policy consideration of affordability and access, coupled with strategies to improve treatment persistence, is essential if these therapies are to meaningfully impact Poland’s obesity burden.