Introduction
Atrial fibrillation (AF) is the most frequent tachyarrhythmia. At present the most effective treatment for it is transcatheter ablation – pulmonary vein isolation (PVI) [1, 2]. However, the procedure is quite long. The duration of the procedure correlates with the risk of complications. Thus, single shot devices were introduced to make the procedure shorter. Balloon technology, especially cryoballoon, is the simplest form of ablation procedure to isolate pulmonary veins [3–6]. However, balloons require typical pulmonary vein (PV) anatomy and cannot be used in all patients. This limitation is not important for two other new technologies: PVAC (pulmonary vein ablation catheter – Medtronic) and nMARQ (Johnson & Johnson). Both are multipoint circular catheters; however, the method of ablation when using them is different. The PVAC is a circular, decapolar 9-Fr bidirectional catheter with nine 3-mm-long electrodes, interelectrode spacing of 3 mm, and a diameter of 25 mm. The PVAC is advanced over a 0.032-inch wire, which is positioned selectively in each PV or its branch to give support and stability to the ablation catheter. The generator provides duty-cycled phased RF energy. The generator allows the operator to select between different ratios of simultaneous unipolar and bipolar energy delivery including unipolar only, 1 : 1, 2 : 1, 4 : 1, or bipolar only. The first generation of PVAC catheters had some limitations especially because of the high risk of silent cerebral ischemia. Thus new generation of the catheter was introduced with gold electrodes, which were used in our study [7]. The nMARQ catheter is a thermocool, circular, decapolar catheter dedicated to the CARTO 3 system only (electrode length 3.5 mm, spacing 4 mm, maximum diameter 8.4 Fr) with a variable circle diameter from 20 to 30 mm. Radiofrequency delivery is in general possible either in a uni- or in a bipolar fashion [8].
Because the technologies are different, it is important to know which one is better in different patients. The aim of our study was to compare in a prospective, randomized study the safety, direct results and periprocedural data of ablation using an nMARQ catheter with the CARTO 3 system, a PVAC catheter used with the EnSite NavX system, or a PVAC catheter only under fluoroscopy control.
Material and methods
A group of 102 (70 male, 32 female, aged 57 ±11 years) consecutive patients qualified for AF ablation were prospectively randomized in a 1 : 1 : 1 ratio to three groups, each consisting of 34 patients. In group 1 ablation was performed with an nMARQ catheter (Jonson & Johnson) together with the 3D CARTO 3 system. In group 2 a PVAC (Medtronic) catheter was used with the 3D EnSite NavX system (St. Jude Medical). In group 3 ablation was done using a PVAC catheter only under fluoroscopy control. Exclusion criteria were as follows: lack of patient agreement, previous linear ablation in the left atrium including antral circular isolation, contraindication for atrial fibrillation ablation, age < 18 years. A comparison of the clinical data of patients in all groups is presented in Table I.
Table I
Patient characteristics (all except hypertension NS)
| Parameter | All patients | Group 1 (nM) | Group 2 (P/E) | Group 3 (P) |
|---|---|---|---|---|
| No. of patients | 102 | 34 | 34 | 34 |
| Male | 70 | 23 | 24 | 23 |
| Age | 57 ±11 | 59 ±9.1 | 58 ±10 | 55 ±13 |
| Weight [kg] | 88.3 ±15.8 | 88.4 ±16.6 | 88.8 ±15.7 | 87.8 ±15.0 |
| Height [cm] | 173 ±9.2 | 172 ±7.7 | 174 ±9.8 | 174 ±9.9 |
| AF history [years] | 5.4 ±4.3 | 5.6 ±4.8 | 4.9 ±3.8 | 5.7 ±4.4 |
| EHRA score | 2.9 ±0.5 | 2.9 ±0.5 | 2.7 ±0.4 | 3.0 ±0.6 |
| CHADS-VASC score | 1.5 ±1.2 | 1.5 ±1.1 | 1.5 ±1.1 | 1.6 ±1.3 |
| Unsuccessful antiarrhythmic drugs | 2.1 ±0.8 | 2.1 ±1.0 | 2.2 ±0.7 | 2.0 ±0.8 |
| LAD [mm] | 42 ±6.6 | 43 ±6.1 | 44 ±5.0 | 40 ±7.8 |
| EF (%) | 60 ±6.8 | 58 ±7.6 | 61 ±6.9 | 62 ±5.0 |
| Paroxysmal AF | 71 | 23 | 22 | 26 |
| Persistent AF: | 31 | 11 | 12 | 8 |
| Long-term persistent AF | 18 | 8 | 5 | 5 |
| Lone AF | 25 | 7 | 7 | 11 |
| Hypertension | 66 | 19 | 28* | 19 |
| Dyslipidemia | 38 | 12 | 17 | 9 |
| Coronary artery disease | 8 | 2 | 2 | 4 |
| Heart failure | 3 | 2 | 1 | 0 |
| Diabetes | 5 | 1 | 0 | 4 |
| Glucose intolerance | 8 | 4 | 2 | 2 |
| Thyroid dysfunction in the history | 23 | 10 | 6 | 7 |
| Hyperuricemia | 3 | 0 | 1 | 2 |
| COPD | 6 | 0 | 2 | 4 |
| OSA | 3 | 1 | 1 | 1 |
| Post-amiodarone pulmonary fibrosis | 1 | 1 | 0 | 0 |
| Stroke in the history | 6 | 3 | 1 | 2 |
| Oncological history | 4 | 1 | 2 | 1 |
| Renal disease | 2 | 0 | 0 | 2 |
| Previous PVI | 5 | 1 | 0 | 4 |
| Previous ablation of CTI | 9 | 2 | 5 | 2 |
Before ablation new oral anticoagulant (NOAC) drugs were discontinued in 34 patients for 48 h before the procedure in accordance with the guidelines applicable during the study [2, 9]. In 48 patients on vitamin K antagonists (VKA) we performed ablation on the drugs and we tried to do it at the therapeutic international normalized ratio (INR) level. The average INR was 2.15 ±0.65 and it was in the therapeutic range between 2.0 and 3.0 in 27 (55%) patients. Nine patients were treated with ASA and 11 patients did not receive anticoagulation before ablation. The CHA2DS2-VASc score in group 1 was 1.5 ±1.1, in group 2 it was 1.5 ±1.1, and in group 3 it was 1.6 ±1.3 (NS). The HAS BLED score was respectively 0.9 ±0.8, 1.1 ±0.8, 1.1 ±0.9 (NS). After ablation all patients received anticoagulation. Patients on NOAC received the drug they used before ablation. Patients on VKA received the same drug or NOAC according to their preference. Patients treated with ASA and those without anticoagulation treatment received NOAC or VKA according to their choice. Patients with a CHA2DS2-VASc score < 2 received the drug for 2–3 months, and the others were treated permanently.
The characteristics of ablation catheters used in the study have been presented in previous publications [7, 8]. Briefly, the nMARQ catheter is a circular catheter and consists of 8 separate thermocool electrodes. Each electrode works independently (may be switched on or off, may have different power and temperature limit). RF application may be performed in unipolar or bipolar mode. Transmurally lesions are verified by a drop of impedance measured at each electrode. The catheter is visualized by the 3D CARTO 3 system. The PVAC catheter is a circular decapolar catheter with 3 mm-long electrodes. In our study, we used the second-generation catheter with gold electrodes. The catheter is inserted using a 0.032-inch guidewire going through the lumen inside the catheter. Ablation is performed using a GENius RF generator (Medtronic Inc) in programmed proportion of uni- and bipolar mode. Typically, this catheter is used only under fluoroscopy control. In our study in group 2 we used the 3D EnSite NavX system. Using this system, we have to change the cable between mapping and ablation parts of the procedure. Also, the guidewire is not seen in the 3D system and manipulation with it requires fluoroscopy.
Ablation procedure
All treatments were performed by an experienced operator in the area of each technological element in the procedures described. In all patients, the diagnostic catheters were introduced into the coronary sinus and into the right ventricle. After that single transseptal puncture was performed using a steerable sheet. If persistent foramen ovale was present, it was used as an attempt into the left atrium. After transseptal puncture the patient received intravenous unfractionated heparin. The dose of heparin was dependent on the activated clotting time (ACT) level (the average peak ACT value was 391 ±109 s). After that, in groups 1 and 2 fast anatomical mapping was performed. In group 3 PV venography was performed to visualize their ostia. After that the isolation of all PV was verified by the nMARQ or PVAC catheter. Ablation at the region near the right pulmonary veins was performed after pacing excluding the phrenic nerve proximity. When a short distance between lines was observed, the circles were connected with ablation lines. If we observed vein potentials during sinus rhythm in the superior vena cava, we isolated this vein with the exception of the region with phrenic nerve palsy.
We compared safety of the procedures in all groups. Complications were subdivided into minor and major (similar to the worldwide survey) [10]. Death, cardiac tamponade, stroke/transient ischemic attack (TIA), pneumothorax, hemothorax, sepsis/abscesses/endocarditis, permanent diaphragmatic paralysis, femoral pseudoaneurysm, artero-venous fistula, valve damage requiring surgery, atrio-esophageal fistulae, and PV stenosis requiring intervention were considered as major complications.
We defined direct success as isolation of all PV. For all procedures we calculated their duration (T), fluoroscopy duration (X), dose area product (DAP), the duration of the applications and the number of applications. All procedures were subdivided inro 3–4 parts: between the femoral venous puncture and the moment when all diagnostic catheters were positioned in correct places, transseptal puncture, angiography (if performed) and proper ablation procedure (to the removal of catheters). We analyzed fluid injection, volume of contrast used during the procedure and the dose of midazolam and fentanyl.
Technical differences between procedures:
Group 1: Diagnostic catheters were introduced using fluoroscopy similar to transseptal puncture and introduction of the nMARQ catheter. Transseptal puncture was performed under intracardiac pressure control; if necessary it was verified with contrast injection. Fast anatomical mapping and PVI were performed under CARTO system navigation to reduce the fluoroscopy. The nMARQ catheter has no guidewire, which decreases contact of the catheter with the atrial wall, when the line is created. It is a thermocool catheter, and thus needs higher attention during ablation at the posterior wall, where we reduce the power and duration of application. Most applications were performed in unipolar mode; however, if the effect was unsatisfactory we used extra bipolar application.
Group 2: Diagnostic catheters were introduced under EnSite NavX control with as low as possible fluoroscopy (in 30 patients totally with no fluoroscopy). Transseptal puncture was performed under fluoroscopy control using pressure control and, if necessary, verified with contrast. Navigation with the PVAC catheter was done with EnSite NavX; however, because the guidewire was not visualized, the introduction and removal of the catheter into/from the vein requires fluoroscopy use. Because the PVAC catheter needs a different cable for diagnostic mapping (when the catheter is visualized by EnSite NavX) and another for ablation (when the catheter is not visualized on the 3D system), before each application the location of the PVAC catheter was checked by brief fluoroscopy before and after cable change.
Group 3: All catheters were introduced and transseptal puncture was performed only with fluoroscopy usage. After transseptal puncture, venous angiography was performed to localize their ostia and to prevent ablation inside the vein. Then, the PVAC catheter was introduced and PVI was performed. The distal part of the PVAC catheter and the necessity to use a guidewire usually make it impossible to perform accessory linear ablation.
The procedure was performed with the understanding and consent of each patient, and with the approval of the local ethics committee (KE/50/2014).
Statistical analysis
The randomization was performed using the program A Randomization Plan from www.randomization.com.
The continuous variables were presented as means and their standard deviations and compared by ANOVA. The discrete variables were presented as numbers and percentages and compared with the χ2 test. P-values less than 0.05 were considered significant.
Results
Lone AF was diagnosed in 25 patients. The most frequent comorbidities increasing the risk of AF recurrence were arterial hypertension (n = 66) and thyroid diseases in the history (n = 23). Other comorbidities are presented in Table I. Paroxysmal form of AF was diagnosed in 71 patients, persistent AF in 13 and long-term persistent in 18. Sinus rhythm at the beginning of the procedure was presented by 63 patients, while in 39 it was atrial fibrillation. At the end of the procedure, 61 patients had sinus rhythm, 40 patients had AF, and left atrial flutter was observed in 1 patient.
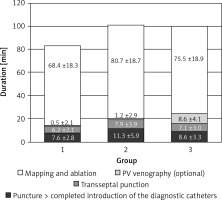
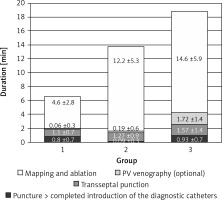
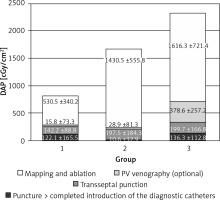
There were a couple of important differences in procedure data between the groups. All data are presented in Table II. Radiological exposure measured as duration of fluoroscopy and DAP were significantly lower during nMARQ ablation (both p < 0.001 vs. groups 2 and 3) and the highest in group 3 (both p < 0.001 vs. group 2). The duration of the procedure was also shorter using the nMARQ catheter (p < 0.001 vs. group 2, NS vs. group 3), and the longest using PVAC with the 3D system (p < 0.001 vs. group 3). The procedure duration and radiological exposure during all parts of ablation are presented in Figures 1–3.
Table II
Ablation parameters
Contrast injection was necessary in 2 patients in group 1 and in 8 patients in group 2 and in all patients in group 3. The average dose of contrast in group 1 was 2.1 ml, in group 2 it was 5.9 ml, and in group 3 it was 44.1 ml (group 1 vs. 2 NS, others p < 0.001). The same trend was found for duration and radiological exposure of this part of the procedure.
The significantly longest duration and the shortest radiological exposure during diagnostic catheter insertion were noted during procedures with the EnSite NavX system (p < 0.001), because in most of these procedures the catheters were introduced only under 3D system control without fluoroscopy. The duration of the mapping and ablation part of the procedures was shorter using CARTO and the nMARQ catheter (p = 0.008 vs. group 2 and NS vs. group 3), and the longest using the PVAC catheter together with the EnSite system (NS vs. group 3). The fluoroscopy duration and DAP were significantly shorter during this part of the procedure using the nMARQ catheter (p = 0.002 vs. groups 2 and 3), and the longest using PVAC only under fluoroscopy control (vs. group 2 p = 0.04 and NS respectively).
Another important difference was the volume of fluid injection during the procedure. The nMARQ is a thermocool catheter and thus the volume of fluid injection using this catheter was significantly higher (p < 0.001 vs. groups 2 and 3). There was no difference between groups 2 and 3. There was no difference between the groups in dose of sedative drugs (midazolam and fentanyl).
Complete isolation was achieved in 400 of 402 PV (99.5%). In 9 vessels verification of the vein was done only anatomically because of the small diameter of the vein (in groups 1–3 respectively: 5, 3 and 1 PV). In 2 patients the right superior pulmonary vein (RSPV) could not be isolated because of the large area of phrenic nerve palsy (in groups 1–3 respectively 0, 1, 1 PV). After isolation of PV linear ablation was performed in 23 patients in group 1 (because of the small distance between lines isolating PV), in 3 patients in group 2 (in the septal region), and in 1 patient in group 3 (mitral line). Lines in group 1: isolation of the posterior segment – 16 patients, line in the left atrial roof – 2 patients, line in the posterior wall – 5 patients, line in the mitral isthmus (after posterior segment isolation) – 1 patient. Isolation of the VCS was performed in group 1 in 1 patient, in group 2 in 9 patients, and in group 3 in 9 patients.
Complications
There were no periprocedural deaths. In our cohort, we observed no cardiac tamponade or neurological complications and during follow-up no atrio-esophageal fistula. However, in 1 older patient after transseptal puncture contrast was injected into the pericardial space. The procedure was terminated and repeated after 2 months with the earlier randomized technique. There were no complications after either procedure. For statistical analysis, we took only data from the second procedure. In 1 patient in group 1 we observed 7–8 mm of pericardial effusion on the following day after the procedure. There was no significant fluid directly after the procedure or after 2 days. In group 2 we observed 2 minor complications: a large hematoma in the puncture region not requiring blood infusion and one episode of transient sinus node dysfunction with junctional rhythm between the 2nd and 3rd day after the procedure with accessory vena cava superior (VCS) isolation. During these 2 days, the patient was treated with corticosteroids and no recurrence of important sinus bradycardia was observed later. In group 3 there was a single complication: in the patient with femoral vein lying directly under the femoral artery we observed pseudoaneurysm and arteriovenous fistula successfully treated conservatively.
Discussion
The purpose of the introduction of circular multi-electrode catheters was to shorten treatment time and reduce the potential fluoroscopy exposure. Studies comparing ablation with single-point and multipoint catheters support the achievement of this objective while maintaining a similar efficacy and safety of ablation [11–14]. McCready et al. [11] presented a multicenter randomized trial comparing PVAC ablation (94 patients with paroxysmal AF) and irrigated single point ablation (92 patients with paroxysmal AF). The mean procedure and fluoroscopy duration were significantly shorter in the PVAC group (140 ±43 min vs. 167 ±42 min and 35 ±16 vs. 42 ±20 min, respectively). In the PVAC group there were 2 strokes, and in the classical group there was 1 clinically important PV stenosis. Lauschke et al. [12] performed a prospective registry with 35 patients randomized 1 : 2 to the nMARQ or single-tip ablation catheter together with a steerable circular mapping catheter. With the nMARQ catheter the ablation time decreased significantly from 18.6 ±13.9 min to 6.3 ±3.0 min (p < 0.05).
To the best of our knowledge, ours is the first prospective randomized trial comparing the first two commercially available multipoint catheters for isolation of the PV using radiofrequency current. Laish-Farkash et al. [15] presented data from a prospective observational study comparing 93 procedures with a PVAC catheter and 82 with an nMARQ catheter. Similar to our study, both technologies have a short procedure duration (94 ±27 and 81 ±18 min). Opposite to our results, the fluoroscopy time was comparable (33 ±13 and 30 ±8.5); however, compared to our results it was quite long. Similar to our cohort, complication rates was comparable using both technologies. Acute and 1-year success rates were similar for both technologies. Concordant with our results, the number of applications and total burning times were shorter with nMARQ. Similar to our observation (not included in results) nMARQ was more suitable for larger atria and PV.
In our study the duration of the procedure with the nMARQ catheter was significantly shorter, being responsible for a shorter portion of the ablation procedure (after transseptal puncture). It was probably due to the guidewire, which is used to stabilize the PVAC catheter. This guidewire makes it difficult to maneuver with the catheter. Furthermore, the nMARQ catheter consists of thermocool electrodes, so the application time is shorter. The main reason for the prolongation of the procedure observed in the case of the PVAC catheter connected to the EnSite system was the need to switch the cable between the mapping period and the application.
Using the 3D system significantly reduced the exposure to fluoroscopy. In the ablation part it was significantly more pronounced in the case of the CARTO system and the nMARQ catheter, probably for the same reasons (switching of the cable and the need to maneuver with the guidewire, which is not seen with the EnSite system). In the case of using mapping 3D systems generally it was not necessary to perform PV angiography, which also reduced the X-ray exposure. In addition, the EnSite system allowed the introduction of diagnostic catheters into the coronary sinus and the right ventricle completely without the use of fluoroscopy [16]. This was associated with a statistically significant but clinically irrelevant (approximately 3 min) extension of the first part of the procedure. The reduction of the fluoroscopy is especially important in patients with oncological history or with increased risk of late oncological complications [17]. In most arrhythmias 3D systems allow ablation to be performed without the use of fluoroscopy except for arrhythmias arising in the left atrium [16, 17]. The reason of that is the necessity to use fluoroscopy for transseptal puncture. However, as stated by Bulava et al. [18], fluoroscopy may be replaced by intracardiac echocardiography. Pulmonary vein isolation without fluoroscopy is also possible in the case of patent foramen ovale [19].
The main differences between the groups were in fluids and contrast injections. Patients treated with the nMARQ catheter received much more fluid than those treated with PVAC. It may be important in patients with heart failure or on dialysis. Patients treated with PVAC need more contrast so ablation with nMARQ should be preferred in patients with renal insufficiency, with thyroid diseases and/or with a history of allergy after contrast injection.
In our previous paper, we reported that isolation of VCS during ablation with a PVAC catheter increases the long-term effectiveness of the treatment [7]. Therefore, in patients undergoing ablation on sinus rhythm VCS was verified and if the vein potentials were observed outside the region of phrenic nerve stimulation, we performed isolation of this vein. Because of much more extensive damage in the case of ablation with the nMARQ catheter, we have not performed routine isolation of the VCS during ablation with this catheter.
The nMARQ catheter caused more extensive damage, and therefore spacing around the left and right pulmonary veins lines was relatively low. For this reason, in many patients it was necessary to isolate the posterior segment or to make a line in the roof or on the posterior wall. In the case of ablation performed with the PVAC catheter, which has the guidewire at the top, usually it was not possible to perform linear ablation.
In accordance with the guidelines, before the ablation procedure VKA were not discontinued [2]. In the case of NOAC the drug was discontinued on the basis of glomerular filtration rate (GFR) without bridging therapy with heparin. In 3 patients after the procedure a large hematoma was observed. In all of them the procedure was performed during treatment with VKA. Since this was the period of our early experience with ablation performed without withdrawal of anticoagulants in these patients after the induction dose of heparin ACT was above 600 s. Since that time, in cases of ablation without the withdrawal of VKA (and now dabigatran also), we have reduced the first dose of heparin. Currently, the reduction of local complications is also due to Z-like sutures.
Ablation with both catheters seems to be safe and effective. However, Vurma et al. [20] in a group of 327 patients treated using the nMARQ catheter reported that there were no serious complications in the first 325 patients, but the last 2 consecutive patients (0.6%) developed atrio-esophageal fistulas and had a fatal outcome. This is the largest published group, and they concluded that the nMARQ catheter is a highly effective tool for treatment of paroxysmal and persistent AF but the occurrence of life-threatening esophageal fistulas is of major concern and requires further investigation. Probably we need to be more careful and more restrictive with the power limit during ablation on the posterior wall of the left atrium.
As we have stated in the title, this work shows only the periprocedural differences between the procedures. Significance of their value is primarily determined by follow-up, which will be the subject of another publication when the data are collected. Since we have found many significant differences between methods during the periprocedural period, we decided to present them in this initial publication.
The second limitation is the relatively small group and one-center study.
In conclusion, the lowest radiological exposure was observed during ablation performed with the nMARQ catheter and CARTO 3 system, and this method should be preferred in patients with comorbidities suggesting reduction of fluoroscopy (e.g. oncological history). 3D systems reduce fluoroscopy duration and the necessity of contrast injection to visualize pulmonary vein ostia. The nMARQ catheter requires injection of a large volume of fluid and should not be preferred in patients with heart failure or severe renal disease.