Atrial fibrillation (AF) is one of the most common cardiac arrhythmias, with studies reporting that it affects more than 33 million people worldwide, and its prevalence is expected to more than double over the next 40 years. AF is associated with important major adverse cardiovascular events, such as heart failure, major stroke, and myocardial infarction, and is also a significant burden on healthcare expenditures [1–4]. Many studies have found that atrial structural remodeling plays an important role in the management of AF. Myocardial fibrosis is the main marker of atrial structural remodeling, which can interrupt the continuity of muscle bundles, interfere with the formation of tight junctions containing connexins, and interfere with cardiac electrical conduction, resulting in slow conduction and conduction block [5]. In addition, coupling between cardiomyocytes and fibroblasts/myofibroblasts can alter cardiomyocyte electrical activity, promote ectopic firing, and promote AF development [6]. Therefore, it is of great clinical significance to clarify the pathogenesis of myocardial fibrosis in AF for the treatment of AF.
Ferroptosis is a kind of cell death mediated by iron-dependent lipid peroxidation. Recent studies have found that ferroptosis can promote liver fibrosis, pulmonary fibrosis and renal fibrosis, and targeted inhibition of ferroptosis can help improve tissue fibrosis [7–9]. However, the role of ferroptosis in myocardial fibrosis in AF has not yet been studied, so this study aimed to explore the relationship between ferroptosis and myocardial fibrosis in AF.
Methods
The subjects of this study were patients with valvular disease who underwent heart valve surgery (Table I). Patients with persistent AF were used as the experimental group (AF group), and patients with sinus rhythm were used as the control group (SR group). The study was conducted in accordance with the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of West China Hospital of Sichuan University (approval No: [2021] No. 143). During the surgery, the left atrial appendage tissue was removed, and one portion was immediately fixed in a 4% paraformaldehyde solution for histological examination. The other part was stored in a freezer at –80°C for western blotting (Wb) analysis (Antibody, Abcam plc, Shanghai, China). The PVDF membrane was incubated with primary antibodies specific for FTH1, GSH, GPX4 and xCT (1 : 2000), GSDMD, cleaved GSDMD, pro-caspase-1, caspase-1, cleaved caspase-1, pro-IL-1β (1 : 1000), and GAPDH (1 : 5000). The results for each protein are representative of three independent repeated measurements. Sirius red staining and Prussian blue staining were used to assess histological fibrosis and ferroptosis changes in the left atrial appendage. Wb detects ferroptosis-related protein expression in the left atrial appendage. The statistical analysis was performed with the software Prism 8 (GraphPad, San Diego, CA, USA). Categorical variables are presented as the number and percentage of patients, and continuous data are shown as mean ± standard deviation. P-values < 0.05 were considered statistically significant. Correlations were calculated using the Pearson or Spearman correlation.
Table I
Clinical characteristics of study group
Results
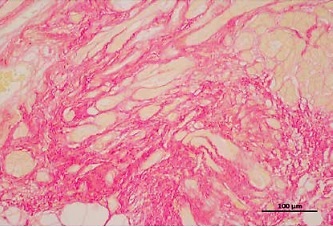
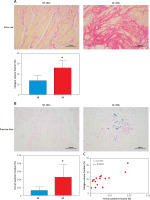
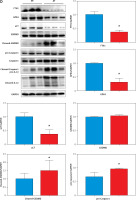
Here, we found that compared with patients in sinus rhythm, patients with AF had significantly increased myocardial fibrosis (SR group 13.35 ±1.81% vs. AF group 25.8 ±2.72%, p = 0.0018) in the left atrial appendage (Figure 1 A). Prussian blue staining found that iron particles in the left atrial appendage tissue of patients with AF increased significantly (SR group 0.012 ±0.003% vs. AF group 0.045 ±0.011%, p = 0.016), indicating that iron metabolism in the myocardium of patients with AF was abnormal (Figure 1 B). There is a certain correlation between the degree of tissue fibrosis and ferroptosis in AF (r = 0.7763, p = 0.0004, Figure 1 C). Wb detection of left atrial appendage tissue confirmed that key proteins related to the regulation of ferroptosis were significantly abnormal in the AF group (Figure 1 D).
Figure 1
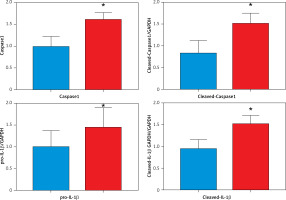
A – Sirius red staining of the left atrial appendage: the collagen ratio was significantly increased in the AF group, indicating that the myocardial fibrosis in the AF group was severe (SR group 13.35 ±1.81% vs. AF group 25.8 ±2.72%, n = 8, p = 0.0018). B – Prussian blue staining showed that iron particles in the left atrial appendage tissue of patients with AF increased significantly, indicating that iron metabolism in the myocardium of patients with AF was abnormal (SR group 0.012 ±0.003% vs. AF group 0.045 ±0.011%, n = 8, p = 0.016). C – There is a certain correlation between the degree of tissue fibrosis and ferroptosis in AF (r = 0.7763, p = 0.0004). D – Expression levels of key regulatory proteins of ferroptosis: FTH1, GPX4, and xCT were significantly downregulated in the AF group, suggesting an important relationship between ferroptosis and AF. The apoptosis-related cytokines (caspase-1, GSDMD, and pro-IL-1β) were significantly elevated in the AF group, suggesting that apoptosis also has an important link with AF
Discussion
Ferroptosis is a newly discovered nonapoptotic regulatory cell death mode that is characterized by iron-dependent lipid peroxidation-mediated cell death. Increasing evidence indicates that ferroptosis mediated by iron metabolism imbalance, abnormal lipid peroxidation, decreased glutathione peroxidase 4 activity and inhibition of the cystine/glutamate transport system are closely related to the occurrence and development of tissue fibrosis [10, 11]. FTH1 (ferritin heavy chain 1) plays an important role in iron absorption and transportation and participates in the formation of stored iron. When the expression of FTH1 decreases, the production of stored iron decreases, which leads to the accumulation of intracellular Fe2+ and induces ferroptosis [12]. When ferroptosis occurs, the intracellular antioxidant glutathione (GSH) is continuously consumed, the activity of glutathione peroxidase 4 (GPX4) decreases, and the oxidation-antioxidant balance is broken. The continuous accumulation of ROS (reactive oxygen species) leads to the loss of cell integrity and cell death after lipid peroxidation occurs in the cell membrane [10]. xCT (SLC7A11) promotes GSH synthesis by mediating cystine uptake and glutamate release, protecting cells from oxidative stress, maintaining cellular redox balance, and preventing lipid peroxidation-induced cell death [13]. These three key regulatory proteins responding to ferroptosis were significantly downregulated in the AF group, suggesting an important relationship between ferroptosis and AF. Meanwhile, we know that caspase-1 cleaves GSDMD (gasdermin D) and cytokines and that the pro-IL-1β precursor is activated by proteolysis to produce its proinflammatory form IL-1β. Visualization of these important cytokines can be achieved by using specific antibodies. Importantly, caspase-1 also cleaves the amino terminal fragment of the key protein GSDMD, causing it to oligomerize and form holes in the cell membrane, leading to cytokine secretion, water intake and eventual cell rupture [14]. These typical apoptosis-related cytokines were significantly elevated in the AF group, suggesting that apoptosis also has an important link with AF.
In conclusion, ferroptosis is a new type of cell death that is neither apoptosis nor necrosis, and has characteristics different from other forms of regulated cell death. However, as a form of death regulated by a variety of substances, it is still unclear which are the most key executive molecules in the process of ferroptosis. At present, there are few studies on the relationship between ferroptosis and cardiovascular disease, and they mainly focus on the mechanism of ischemia-reperfusion injury. However, regarding the pathological mechanism of myocardial fibrosis, there is little research on ferroptosis, especially the mechanism by which ferroptosis regulates myocardial fibrosis in AF. We have preliminarily confirmed that ferroptosis is related to myocardial fibrosis in AF, which will provide theoretical support for targeting the inhibition of myocardial fibrosis and the treatment of AF.