Introduction
Atrial fibrillation (AF) is one of the most common supraventricular arrhythmias, affecting 1.0–1.5% of the world population [1]. The most serious complication of AF is a thromboembolic event such as stroke, transient ischemic attack (TIA), or systemic arterial embolism [2]. Atrial fibrillation contributes to thromboembolism by way of various mechanisms. The most significant series of mechanical events leading to thromboembolism begins when the left atrium of the heart becomes congested with blood. This cardiac congestion leads to diminished functionality, which can trigger the formation of a thrombus created from the congested material. Once formed, arrhythmic events can loosen the thrombus and carry it into the bloodstream, where it can become lodged in a blood vessel, causing a blockage of blood flow, leading to a stroke [3, 4]. The presence of AF is associated with a 5-fold increase in the incidence of ischemic stroke in patients with AF [5–7]. Effective prophylaxis with anticoagulants is an important factor in preventing stroke in patients with AF [6–8]. It is estimated that the annual stroke incidence in AF patients not receiving anticoagulation therapy accounts for 4.9–5.7% of cases [9, 10]. Undiagnosed AF may cause up to 30% of all cardiogenic strokes [6, 8]. In AF patients with stroke, the prognosis is worse compared to patients with a sinus rhythm, with in-hospital mortality rates as high as 30% [6, 11–13]. The risk of annual mortality and disability after stroke in patients with AF is two times higher than in the population without AF [7].
The aim of the study was to compare in-hospital mortality between ischemic stroke patients diagnosed with AF de novo (during hospitalization due to stroke) and ischemic stroke patients diagnosed with AF prior to hospitalization due to stroke.
Material and methods
Study group
A retrospective analysis was conducted on acute stroke patients hospitalized in a tertiary center during the years 2013 and 2014. Patients with cerebral hemorrhage and TIA were not included in this study.
Stroke was diagnosed by clinical presentation as an episode of neurological dysfunction lasting more than 24 h and imaging studies (head computed tomography or magnetic resonance imaging).
Two groups of study patients were identified, using criteria established by the European Society of Cardiology (ESC): 1) new-AF group: these patients had no previous history of arrhythmia but AF was diagnosed de novo, concurrently, during their hospital stay for stroke; 2) previous-AF group: these patients had been diagnosed with AF before hospitalization for stroke.
In addition to ESC diagnostic guidelines, AF diagnoses were based on an electrocardiogram showing irregular atrial rhythm lasting 30 s or longer [14].
We compared baseline characteristics and outcomes between these two groups.
The study was approved by the ethics committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards.
Analyzed data
The following data was analyzed: demographic data (age, sex), comorbidities, diagnostic study results, and hospital course. Risk factors for stroke, such as hypertension, diabetes mellitus, heart disease, and current smoking were assessed.
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg in repeated tests, any use of antihypertensive drugs or self-reported history.
Diabetes mellitus was defined as a fasting blood glucose level greater than 126 mg/dl after a minimum of two tests or a glucose level greater than 200 mg/dl any time during the day, self-reported patient history or use of anti-diabetic medications [8]. Heart disease was determined by a previous history of coronary artery disease or heart failure.
The extent of ischemia was evaluated by head computed tomography or magnetic resonance imaging. Head imaging was performed at admission and repeated during hospitalization.
Hemorrhagic transformation was defined as a focus of hemorrhage on brain CT or MRI.
Doppler ultrasound was used to evaluate extracranial cerebral circulation.
In all patients, resting 12-lead electrocardiography was performed at admission and discharge.
In most patients (n = 1489), Holter monitoring was conducted for 24 h following admission.
Also, transthoracic echocardiography was conducted on selected patients (n = 1230).
Stroke severity was evaluated in accordance with the National Institutes of Health Stroke Scale (NIHSS) at admission. For the assessment of stroke risk in AF patients, the CHADS2 scale was applied accounting for previous history of stroke, transient ischemic attack, systemic thromboembolism, age ≥ 75, hypertension, diabetes, and heart failure, together with the CHA2DS2VASc scale with additional points for female sex, age 65–74 and vascular disease. A score of 0 on both scales denotes low risk, 1 point intermediate risk, and ≥ 2 points high risk of thromboembolic events. Evaluation using both CHADS2 and CHA2DS2VASc scales was conducted twice, without considering the current stroke event (pre-stroke CHADS2 and CHA2DS2VASc) as well as taking into account the current episode (post-stroke CHADS2 and CHA2DS2-VASc).
Statistical analysis
Categorical data were presented as the number of patients and for the ordinal data means with SD were used. To determine differences between the groups the χ2 test was applied for categorical data (with the exception of cases of occurrence numbers less than 5 in contingency tables where Fisher’s exact test was performed) and Student’s t-test was used for verification of hypothesis of equality of the means for continuous data. Statistical significance was considered for p-values lower than 0.05 calculated for two-tailed tests. Odds ratio parameters with 95% confidence intervals and corresponding p-values were calculated, separately for both groups, using a logistic regression model to assess the influence of the predictors on death during hospitalization. A univariate analysis was followed by multivariate analysis performed for a selected subset of variables considered as the most important, including the ones found to be statistically significant in the univariate model for both groups. Statistical analyses were performed using R: A language and environment for statistical computing, R Development Core Team (2017), http://www.R-project.org/. For the cases of zero cells the model was run using the Bayesian framework (namely the “bayesglm” function from the “arm” package), which was considered to be more appropriate and leading to less extreme estimates. However, there were no differences in terms of statistical significance between the Bayesian and the standard (“glm” function) approach.
Results
Baseline characteristics
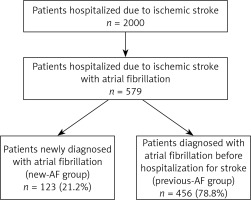
Of the entire study population of 2,000 ischemic stroke patients, AF was diagnosed in 579 (29%), who were subject to further study. Of these AF patients, the mean age was 78.6 years and the majority were over 74 (408 patients, 70.4%). This group included 358 (61.8%) females.
Within the group of 579 patients with AF, arrhythmia was newly diagnosed in 123 (21.2%) individuals (new-AF group), while 456 patients (78.8%) had a previous history of AF (previous-AF group) (Figure 1). The new-AF group included 77 females (66.6%), while the previous-AF group included 281 females (61.2%, p = 0.93).
Among patients with stroke and AF (n = 579), the most common coexisting pathologies were hypertension (471; 81.3%), coronary artery disease (278; 48%), heart failure (167; 28.8%) and diabetes (160; 27.6%). Table I shows clinical characteristics of new-AF and previous-AF groups.
Table I
Clinical characteristics of the study group
[i] Continuous and ordinal variables are shown as mean (SD) unless otherwise indicated. P values are given for differences between new-AF and previous-AF groups. A p-value of < 0.05 is considered statistically significant. AF – atrial fibrillation, GFR – glomerular filtration rate, HGB – hemoglobin, LA – left atrium, LVEF – left ventricular ejection fraction, NIHSS – National Institutes of Health Stroke Scale, TIA – transient ischemic attack.
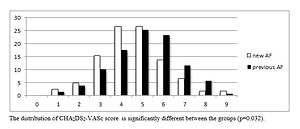
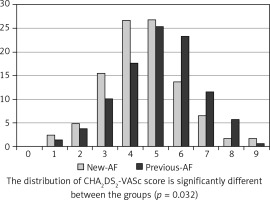
The pre-hospital stroke risk expressed by the CHA2DS2VASc score was, on average, higher in the previous-AF group than in the new-AF group (5.1 vs. 4.6, p = 0.001). In the previous-AF group, high stroke risk rate expressed by a high pre-stroke CHA2DS2VASc score was higher than in the new-AF group. The difference was statistically significant for patients with a CHA2DS2VASc score > 5 points (Table II).
Table II
Risk of thromboembolism of the study group
Figure 2 shows pre-stroke CHA2DS2VASc scores for the entire study group.
Figure 2
Patients in the study group at the time of ischemic stroke according to CHA2DS2-VASc score. CHA2DS2-VASc score is calculated at the time of the ischemic event and scoring does not include the current event
Stroke severity, determined by the mean NIHSS score, was higher in the group with new AF than in the group with previous AF (7.2 vs. 3.7, p < 0.001).
In the new-AF group, 14 (11.4%) patients received antiplatelet therapy before hospitalization, but there were no patients who received oral anticoagulation, whereas in the previous-AF group, 83 (18.2%) patients received anticoagulants (all patients were treated with vitamin K antagonists), 78 (17.1%) patients received antiplatelet agents, and 21 (4.6%) patients did not receive any stroke prophylaxis.
Within the combined study population, thrombolysis was used in 44 patients (7.6%): 15 (12.2%) from the new-AF group and 29 (6.4%) from the previous-AF group (p < 0.048). In the study group thrombectomy was not used. Hemorrhagic transformation during hospitalization was observed in 5 patients (4.1%) from the new-AF group and in 13 (2.9%) from the previous-AF group (p = 0.692).
The endpoint of an in-hospital death was more common in the new-AF group (13 patients; 10.6%) than in the previous-AF group (16 patients; 3.5%) (p = 0.003).
Univariate analyses of predictors of death at hospitalization in the new-AF group and the previous-AF group
In univariate analyses, the risk factors of death were identified and compared between groups. In the new-AF group, the NIHSS score at admission proved to be the most significant indicator of death during hospitalization. In the previous-AF group, the most significant risk factors for mortality included the NIHSS score at admission and multiple ischemic foci (Table III).
Table III
Univariate analyses of predictors of death at hospitalization in both groups
Multivariate analyses of predictors of death at hospitalization in the new-AF group and the previous-AF group
Table IV shows predisposing factors of mortality identified in multivariate analyses. It was observed that the NIHSS score at admission in the new-AF group was associated with higher mortality, while in the previous-AF group the NIHSS score at admission and multiple ischemic foci presented higher risk for in-hospital mortality.
Table IV
Multivariate analyses of predictors of death at hospitalization in both groups
Discussion
Atrial fibrillation is a common cause of thromboembolic incidents, including stroke. Arrhythmia is often asymptomatic, but lack of symptoms does not reduce the risk of stroke, even when compared to symptomatic AF [15–17]. In patients known to have AF, the risk of stroke can be minimized with anticoagulation therapy [7, 18, 19]. Thus, in asymptomatic patients not undergoing anticoagulation therapy, the stroke risk is potentially higher than in symptomatic individuals receiving treatment for AF.
In this study, we determined that among acute ischemic stroke patients with AF, the population with newly diagnosed AF was 21%. Clinical study of the incidence of AF de novo in stroke patients is limited. The Jaakkola et al. study of a group of 3,623 patients showed that AF de novo was observed in 21.9% of stroke patients and in 16.4% of TIA patients [18]. A similar rate was noted in other studies, in which AF was diagnosed in 22.2–22.6% of stroke patients [19, 20]. Borowsky et al. reported AF de novo in 18% of 856 patients [21].
In this study on a population of patients with an ischemic stroke, most of them (70.5%) were over 74 years of age. Their collective pre-stroke CHA2DS2VASc mean score was 4.9 points and CHADS2 was 2.5 points.
The studied group consisted mainly of elderly individuals with high risk of thromboembolic events. In a separate study, Borowsky et al. reported CHA2DS2-VASc ≥ 2 points in 89% of patients with stroke and AF. In this study, the new-AF group did not differ from the previous-AF group in age and sex. However, previous-AF group members were more likely than new-AF group members to suffer from hypertension, coronary artery disease, and heart failure, and have a history of thromboembolism. Mean pre-stroke CHADS2 and CHA2DS2VASc scores were higher in the previous-AF than in the new-AF group.
Interestingly, when CHA2DS2VASc scores rea-ched 5 points and higher, the higher scores led to statistically significant differences in the outcome. Therefore, in the previous-AF group, very high stroke risk was more prevalent than in the new-AF group.
However, a higher mean NIHSS score was noted in the new-AF group than in the previous-AF group. During their hospital stay, 10.6% of patients in the new-AF group and 3.5% in the previous-AF group died, representing an overall total of 5% of patients with coexisting stroke and AF.
Our findings are in accordance with reports by other authors. In a group of 4278 ischemic stroke patients, AF was associated with increased in-hospital mortality in women but not in men compared with patients without AF [22]. Based on the Austrian Stroke Registry, the in-hospital mortality due to ischemic stroke with AF was 25%, while it was 14% for patients without arrhythmia [23]. In the Austrian group of 25,319 patients with an ischemic stroke and AF, the in-hospital mortality rate was 14.1%, while it was 6.2% when AF was not present [24]. Borowsky et al. reported even higher mortality of 15% in patients newly diagnosed with AF [21]. In our study the new AF was probably not new, but newly detected. Most likely, it was a non-paroxysmal AF, which is more often asymptomatic and less frequently detected.
Deguchi et al. suggested that the type of atrial fibrillation affects stroke severity and clinical outcomes following cerebral infarction. In a study of 9293 patients with cardioembolic stroke and AF, those with persistent AF had significantly higher stroke severity on admission than those with paroxysmal AF, and persistent AF was a factor contributing to the in-hospital mortality [25].
In this study, we identified predisposing risk factors of in-hospital mortality in patients with stroke and atrial fibrillation de novo as well as in patients with a previous diagnosis of this arrhythmia. In the previous-AF group, we found in univariate analysis that multiple ischemic foci and high NIHSS score were associated with higher mortality. Interestingly, age and a pre-stroke CHA2DS2-VASc score > 5 were associated with lower risk.
In the univariate analysis of the new-AF group, a high NIHSS score at admission was associated with higher mortality, while high stroke risk resulted in lower mortality. In multivariate analysis, we confirmed that a high NIHSS score at admission was a risk factor of high mortality in the new-AF group, while a high NIHSS score at admission and multiple ischemic foci were risk factors of in-hospital mortality in the previous-AF group. In the multivariate analysis, it was not confirmed that high stroke risk expressed on the CHA2DS2VASc scale had an impact on the in-hospital mortality of either the new-AF group or the previous-AF group.
Most published research on the subject at this time indicates that a high stroke risk is a poor prognostic factor for a long-term follow-up of stroke patients [26, 27]. It is possible that a prolonged observation affected the results. In this study, we found that the most predictive factors of in-hospital mortality in stroke patients with AF included neurological status manifested by a NIHSS score at admission as well as multiple ischemic foci. These observations have been corroborated by other authors [27].
The ESC guidelines recommend regular assessment of rhythm in screening for occult AF in all patients over the age of 65 [14]. This study shows that patients with stroke and newly diagnosed AF have poor prognosis regardless of age and comorbidities. Therefore, it is justified to screen for occult AF in young patients without concurrent medical conditions since asymptomatic AF significantly increases the risk of stroke [15].
In the Cryptogenic Stroke and Underlying Atrial Fibrillation (CRYSTAL-AF) study conducted on patients with cryptogenic stroke, those receiving standard care were compared with those fitted with an implantable loop recorder. In follow-up research conducted 6 months after enrollment in the study, 8.9% were diagnosed with AF for the first time. At 12-months follow-up, 12.4% were newly diagnosed with AF [28].
In the Asymptomatic Stroke and Atrial Fibrillation Evaluation in Pacemaker Patients (ASSERT) trial, no definite temporal relationship was found between short asymptomatic episodes of AF and stroke [29]. The role of AF in the pathogenesis of thromboembolism is well established; however, other mechanisms can be present in patients with arrhythmia that leads to stroke.
There are several limitations of our study. As is the case for all retrospective studies, there exist potential unidentified confounders. We could not adjust for an individual-level socioeconomic status, form of AF and burden of AF in the study group. Our data source could not ascertain the date of thromboembolic prevention and other treatment before hospitalization. Data on anticoagulant and antiplatelet treatment in a significant number of participants were unknown and could not be obtained due to the retrospective nature of the study. Another limitation of our study is that data on some of the variables were not available for all of the patients (i.e. ultrasound doppler of carotid arteries, echocardiography).
In conclusion, newly diagnosed AF in ischemic stroke patients significantly worsens short-term prognosis compared to patients with earlier diagnosed AF. Stroke severity on admission was found to be the most significant predisposing risk factor for the in-hospital mortality for all ischemic stroke patients with AF.
Our results emphasize the importance of detecting latent atrial fibrillation, to improve stroke outcomes through effective prevention with anticoagulation therapy.