Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease, a spectrum of clinicopathologic conditions ranging from simple steatosis to steatohepatitis and cirrhosis [1]. The prevalence of NAFLD in the adult population ranges from 20% to 33%, and is drastically increased up to 75% in the setting of obesity or type 2 diabetes mellitus [2]. The NAFLD is growing into a worldwide health burden [3, 4]; unfortunately, there are few beneficial treatment options other than those centering upon lifestyle interventions such as physical exercise and dietary changes [5, 6]. This nevertheless is difficult for most patients in the long term. Thus, there has been a need for a medical means of convenient and effective management of NAFLD.
Since the proposal of the “gut-liver axis” concept by Marshall in 2008, the role of the intestinal barrier in the pathogenesis of NAFLD has gradually become acknowledged by researchers [7]. A series of studies have suggested that structural changes in the intestinal flora, overgrowth of intestinal bacteria and intestinal endotoxemia have all contributed to the development of NAFLD, whereas restoring the intestinal microflora balance and reducing the intestinal permeability may ameliorate the progression of NAFLD [8–10].
Probiotics such as Lactobacillus paracasei N1115 (N1115) are live microorganisms with health benefits to the hosts when consumed in adequate amounts [11]. It has been proposed that probiotics can be used as a potential treatment of NAFLD by improving epithelial barrier functions, reducing intestinal inflammation, and regulating the gut-associated immune system [12–14]. Prebiotics such as fructooligosaccharides (FOS) on the other hand are nonviable carbohydrates that positively affect the hosts by selectively modifying the growth and activity of intestinal bacteria [15]. Synbiotics, as their name states, are a combination of probiotics and prebiotics.
Despite the notion that probiotics, prebiotics and the combinational synbiotics may prevent and treat NAFLD, there has been limited evidence documented by the literature [14]. Herein, we conducted a study to evaluate the effect of N1115 and FOS in NAFLD. Using the C57BL/6 mouse model, we analyzed the histopathogenesis and molecular events such as inflammatory response, insulin resistance, intestinal permeability and its related protein expression changes. Thereby, we hoped to further our understanding of the mechanistic basis of NAFLD, and also provide direct preclinical evidence to support the use of probiotics, prebiotics, and synbiotics in the epidemiological prevention and clinical management of NAFLD.
Material and methods
Materials
High-fat diet (HFD) was purchased from Jiangsu biological Ltd. (Jiangsu, China). Lactobacillus paracasei N1115 (N1115) was produced from fermented dairy products according to the Inner Mongolia traditional method. Antibodies against claudin-1, occludin-1, p38, and phosphor (p)-p38 were acquired from Abcam plc., MA, USA. Enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor (TNF)-α, interleukin (IL)-1β, insulin (IR), lipopolysaccharides (LPS) and monoamine oxidase (MAO) were purchased from Cusabio Biotech Co. (Wuhan, China).
Animal model
C57BL/6 mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) under the protocol SCXK 2012-0001. After feeding with a normal diet for 21 days, 50 4-week old male mice were randomized into 5 groups: control group, model group, N1115 group, FOS group and the combination (coadministration of N1115 and FOS) group. The control group was fed with a normal diet (ND). The model group was administered a high-fat diet. The N1115 group was fed with a high-fat diet with N1115 (2.2 × 109 CFU/ml in normal saline, 0.5 ml/day). The FOS group was administered a high-fat diet with FOS (4 g/kg/day). The combination group was treated with a high-fat diet with N1115 and FOS (doses indicated as above). The experimental procedure was continued for 16 weeks with weekly body weight measurement. Intraperitoneal glucose tolerance tests (IPGTT) were performed after 16 weeks of feeding. All experiments were approved by the Ethics Committee of Zhengzhou University.
Histopathologic examination
Tissue specimens obtained from liver and intestine were fixed in 4% formaldehyde for 24 h prior to paraffin embedding and microtome sectioning. Hematoxylin and eosin (H + E) staining was performed for the histopathologic analysis of tissue sections. Additionally, one portion of liver tissue was snap frozen in liquid nitrogen and prepared for Sudan III fat staining after cryosectioning.
Serological measurement
Blood collected from eye enucleation was centrifuged at 2000 rpm at 4°C. The plasma was analyzed for total triglyceride (TG) and total cholesterol (TC) with a reagent kit from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). ELISA kits (Cusabio Biotech Co., Wuhan, China) were used to quantify TNF-α, IL-1β, IR, LPS and MAO in the peripheral blood and TNF-α and IL-1β in the liver tissue according to the manufacturer’s instructions. Peripheral blood glucose concentration was determined using an automated glycemia reader (Accu-Chek Active Blood Glucose Meter, ROCHE Inc., Germany). Homeostatic model assessment of insulin resistance (HOMA-IR) was performed with the following formula: (immuno-reactive insulin (mIU/l) × fasting blood sugar (mmol/l) ÷ 22.5) according to the published method [16].
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from the liver or ileum tissue with RNAiso Plus, and reverse transcribed into cDNA. Real-time PCR was performed using the SYBR Green Real-time PCR Master Mix kits (TOYOBO Co. Ltd., Osaka, Japan) with the PCR primers listed in Table I. The specificity of PCR primers was validated with BLAST analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. In addition, all PCR products were confirmed by melting curve analysis to distinguish them from non-specific products or primer dimers. PCR products were resolved by agarose gel electrophoresis. Gene expression was illustrated by the ratio of the target gene to GAPDH.
Table I
Primers used in real-time PCR
Western blotting
Total proteins were extracted from the liver or ileum by homogenization in tissue lysis buffer. Protein concentration was determined by the BCA method. Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and blotted onto polyvinyl difluoride (PVDF) membranes. Proteins were detected by incubating with the indicated antibodies. The Western blotting results were quantified with ImageJ (National Institutes of Health, USA).
Immunohistochemistry
Immunohistochemistry followed the standard procedure. In brief, mouse liver tissue sections were antigen retrieved by citrate. 3% hydrogen peroxide was used to block endogenous peroxidase and goat serum was used to block non-specific reactions. The primary antibody reactions were carried out overnight at 4°C. The staining was visualized with diaminobenzidine (DAB) after incubating with secondary antibodies.
Results
N1115, FOS and their combination alleviate hepatic steatosis, inflammation, and cirrhosis
The NAFLD was histologically classified into two categories: simple hepatocellular steatosis and nonalcoholic steatohepatitis. The latter is characterized by steatosis, inflammation and cell injury [17]. We first assessed the histopathology of our NAFLD model with H + E and Sudan III staining. We found that high-fat diet (HFD)-fed mice had increased hepatic lipid accumulation, consistent with nonalcoholic steatosis. In comparison, HFD with N1115, FOS or their synbiotic combination significantly alleviated the steatosis in our C57BL/6 mice. The combination of N1115 with FOS appeared to have the most protective effects against the development of steatosis in our histologic evaluation (Figure 1 A).
Figure 1
Lactobacillus paracasei N1115, fructooligosaccharides (FOS) and the combinational synbiotics alleviate hepatic steatosis, inflammation, and cirrhosis. A – Representative H + E and Sudan III fat staining of liver tissue sections. Data represent 10 individual slides. Original magnification, 100×. ELISA quantification of IL-1β in serum (B), IL-1β in liver (C), TNF-α in serum (D), TNF-α in liver (E), and monoamine oxidase (MAO) in serum (F)
▴P < 0.05, compared to ND. ✦P < 0.05, compared to HFD. ND – normal diet, HFD – high-fat diet, L – N1115, F – FOS.
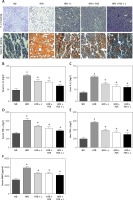
Further, we evaluated the systemic and hepatic inflammations by quantifying the serum and hepatic inflammatory factors including Interleukin-1β and TNF-α. These factors are predominantly produced by the hepatocytes and Kupffer cells. Our HFD group showed elevated concentrations in the serum and hepatic IL-1β and TNF-α (Figures 1 B–E). HFD-induced TNF-α activation was significantly alleviated with the dietary supplementation of N1115, FOS or synbiotics (Figures 1 D, E). Repeatedly, we observed more pronounced effects in the combinational synbiotics group. Moreover, we examined the serum monoamine oxidases (MAO) as a parameter of liver fibrosis or cirrhosis. The serum levels of MAO were significantly decreased by the pharmaceutical interventions (Figure 1 F).
N1115, FOS and their combination reduce serum lipids and overcome insulin resistance
The NAFLD has been associated with the metabolic syndrome, a constellation of health conditions including abdominal obesity, hypertriglyceridemia, decreased high-density lipoprotein (HDL), hyperglycemia and hypertension. Our animal model data showed that a HFD diet led to elevated total triglyceride (TG) and cholesterol (TC) in the peripheral blood. Dietary supplementation of N1115, FOS, or synbiotics significantly reversed the dyslipidemia induced by the HFD (Figures 2 A, B).
Figure 2
N1115, FOS, and the combinational synbiotics reduce serum lipids and overcome insulin resistance. ELISA quantification of total cholesterol (TC) (A) and triglyceride (TG) (B) in serum. C – Fasting blood glucose measurement. D – Fasting blood insulin. E – Intraperitoneal glucose tolerance tests (IPGTT). F – Scores of HOMA-IR. G – Insulin receptor mRNA in liver. H – Insulin receptor substrate-1 mRNA in liver
▴P < 0.05, compared to ND. ✦P < 0.05, compared to HFD. ND – normal diet, HFD – high-fat diet, L – N1115, F – FOS.
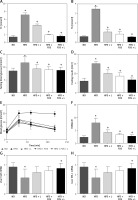
Further, with regards to fasting blood glucose and insulin, we observed that the addition of N1115, FOS, or synbiotics decreased the fasting blood glucose (Figure 2 C) and insulin (Figure 2 D) to the base levels. Intraperitoneal glucose tolerance tests (IPGTT) showed that the effect on glucose metabolism was evident in as short as 30 min and persisted for at least 120 min (Figure 2 E). Homeostatic model assessment of insulin resistance (HOMA-IR) showed a high score in the HFD group, indicating impaired glucose clearance. Dietary interventions with N1115, FOS or synbiotics effectively decreased the insulin resistance (Figures 2 E, F). Next, we analyzed the transcriptional regulation of insulin receptor (InsR) and insulin receptor substrate (IRS)-1 as a direct measure to quantify insulin resistance. We noted the abrogation of transcriptional suppression of InsR and IRS-1 by HFD in the groups of FOS and synbiotics (Figures 2 G, H).
N1115, FOS and synbiotics suppress the NAFLD associated inflammation
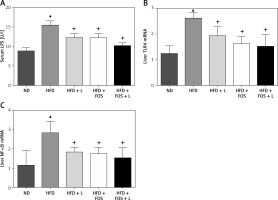
Intracellular signaling transduction has been suggested as an integration of many inflammatory factors contributing to the development of NAFLD. The Toll-like receptor (TLR) 4-lipopolysaccharides (LPS) pathway is one of the key pathways governing the inflammation associated with NAFLD. TLR4 serves as the receptor for LPS to induce hepatic inflammation. Further, NF-κB is a transcription factor regulating a variety of cytokines closely related to the systemic inflammation. In this study, we investigated the transcriptional regulation of three genes in our animal model. Real-time PCR results showed that LPS, TLR4 and NF-κB were transcriptionally regulated by the HFD, and this regulation was canceled by the pharmaceutical actions of N1115, FOS and synbiotics (Figures 3 A–C).
Figure 3
N1115, FOS and the combinational synbiotics suppress the NAFLD associated inflammation. A – ELISA quantification of serum lipopolysaccharides. B – Real-time PCR quantification of Toll-like receptor (TLR) 4 in liver. C – Real-time PCR quantification of nuclear factor (NF)-kB in liver
▴P < 0.05, compared to ND. ✦P < 0.05, compared to HFD. ND – normal diet, HFD – high-fat diet, L – N1115, F – FOS.
N1115, FOS, and synbiotics improve the intestinal barrier functions
The intestinal barrier fulfills protective functions against enteric organisms, bacterial products, and toxins. The progression of NAFLD has been related to the intestinal barrier malfunction such as increased intestinal permeability. Our H + E stained mouse intestinal tissue showed common histopathologic changes of NAFLD such as edema, ischemia, and atrophy in the HFD-fed group (Figure 4 A). Treatment with N1115, FOS and synbiotics appeared to restore the normal intestinal mucosa integrity and histology.
Figure 4
N1115, FOS, and the combinational synbiotics improve the intestinal barrier functions. A – Representative histopathologic morphologies observed in the mice intestine. Original magnification 100×. B – Western blotting analysis of p-p38, p38, occludin-1 and claudin-1. β-actin was used as loading control. Protein expression was quantified using ImageJ and presented as relative numerical values. P-p38 was normalized with p38 and β-actin. Occludin-1 and claudin-1 were normalized with β-actin
ND – normal diet, HFD – high-fat diet, L – N1115, F – FOS.
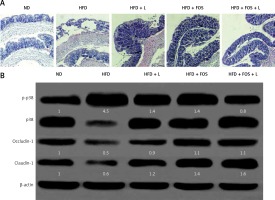
At the molecular level, we studied two tight junction proteins, occludin-1 and claudin-1. These two proteins are essential for preserving the intestinal barrier functions. In addition, the p38 mitogen-activated protein kinase (MAPK) signaling pathway regulates the molecular composition of tight junction complexes and intestinal permeability. We found that the HFD induced significant p38 MAPK phosphorylation and activation, and was associated with reduced expression of occludin-1 and claudin-1. Treating the animals with N1115, FOS and synbiotics appeared to reverse these molecular changes.
Discussion
In recent years, NAFLD has been a growing health concern of modern society due to its related morbidity and mortality [18, 19]. Despite the increased awareness of this condition, there has been limited progress in the clinical management of NAFLD [20]. Most physicians recommend lifestyle modifications such as weight loss, decreased calorie intake and exercise. There has not been a consensus for a definite pharmacotherapy.
Synbiotics are a combination of the two dietary supplements probiotics and prebiotics. The synergistic benefits have been documented for disease conditions such as obesity and diabetes. A study by Kelishadi et al. evaluated the anti-inflammatory effects of synbiotics in the overweight pediatric population [21]. Their data suggested that therapy with synbiotics had an impact on inflammatory factors. In another study, a cohort of patients with insulin resistance was managed with synbiotic agents containing seven strains of probiotics and FOS. The group treated with synbiotics had a remarkable improvement in fasting blood glucose and insulin resistance [22].
Nonetheless, the effect of synbiotics on NAFLD is in the trial stage of research. A synbiotic supplement was tested in patients with NAFLD with a focus on hepatic fibrosis, liver enzymes, and inflammatory markers [23]. Their data showed the inhibition of NF-κB production. In addition to agreeing with their results on NF-κB, our synbiotics had a further impact on TNF-α production [24]. Combinational use of probiotics and prebiotics appeared to have an additive to possible synergic effect on the inflammatory markers. Unfortunately, our study did not follow a dose-effect model to allow a direct measure of pharmacological synergy (such as by calculating the combination index).
Admittedly, studies done in experimental rodents may not entirely recapitulate the human histopathogenesis. The fat diet induced animal model nonetheless has been widely accepted to study NAFLD, particularly useful for the testing of novel therapeutic agents. Our C57BL mice are an ob/ob model with a gene mutation causing leptin deficiency [25]. It is capable of simulating a variety of NAFLD conditions such as high TG, high TC, obesity, and hepatic steatosis. However, ob/ob mice require a ‘second hit’ such as LPS to trigger the progression from steatosis to steatohepatitis instead of the spontaneous progression seen in the human population [25]. Detection of MAO, IL-1β, and TNF-α in the current study has confirmed that this LPS-triggered ‘second hit’ event had likely occurred in our mouse model.
The LPS is released from the intestinal gram-negative bacteria and is an important agent of stimulating strong inflammatory responses and causing liver damage. TLR-4 is the receptor to LPS expressed by a variety of hepatic cells. Activation of TLR-4 leads to the production of pro-inflammatory, antiviral and anti-bacterial cytokines [26]. Many studies have suggested that the LPS/TLR4 signaling pathway mediates inflammation, oxidative stress, insulin resistance and liver fibrosis, and is the key in the histopathogenesis of NAFLD [27, 28].
Lastly, the progression of liver damage in NAFLD is associated with increased intestinal permeability. The P38 MAPK signal pathway alters the molecular composition of tight junction complexes through regulating the expression of tight junction proteins [29–31]. By regulating the epithelial tight junction components, p38 MAPK has been shown to contribute to the repair of injured gastric epithelium [32–35]. For example, Oshima et al. [33] and Kevil et al. [34] suggested that p38 MAPK enhanced the expression of epithelial mucosal tight junction proteins to decrease the intestinal permeability, thereby reducing the entry of toxins to the intestinal wall and bloodstream. Our study likewise found that dietary intervention with N1115, FOS and synbiotics increased the expression of tight junction proteins occludin-1 and claudin-1, likely through regulation (phosphorylation and activation) of p38 MAPK. This likely better preserved the intestinal barrier functions and lowered the entry of LPS from the intestine to the liver and consequently inhibited activation of the LPS-TLR4 signaling pathway.
In conclusion, collectively, our preclinical study with the animal model shows that N1115, FOS, and the combinational synbiotics are effective in the prevention and treatment of NAFLD. Our data support the translation of these agents into a clinical evaluation in human subjects with NAFLD and/or associated risk factors.


