Introduction
The survival rate of women with a variety of cancers has increased significantly with improvements in cancer treatment, including early detection and effective management [1]. An increasing number of reproductive-age female cancer survivors require quality-of-life care, covering the preservation of their fecundity, from clinicians and healthcare professionals.
Female reproductive function is represented by cyclic ovarian follicular development. Ovarian follicles are composed of oocytes and granulosa cells (G-cells), which are germ and somatic cells, respectively [2]. Although this machinery should work together in order to achieve physiological follicular development and oocyte maturation, different chemotherapeutic agents with different mechanisms of action may affect each component cell in a different way.
Cisplatin and paclitaxel are the most widely used chemotherapeutic agents for the treatment of gynaecological malignancies, and their mechanisms of action against ovarian cancer cells have been thoroughly investigated. Cisplatin interferes with DNA replication, which kills the fastest proliferating cells [3]. Paclitaxel’s mechanism of action involves the interference with the normal breakdown of microtubules during cell division [3]. Paclitaxel acts as a mitotic inhibitor by disrupting microtubule function [4] and is able to alter mitochondrial membrane permeability in vitro [5].
Some recent studies have focused on the effects of their in vivo administration that caused primordial follicular apoptosis observed from a cross-section of the whole ovarian tissue [3]. However, the magnitude of reproductive toxicological potential affecting normal ovarian follicular development is still unknown. This issue is critical in that clinicians can cite these data, if available, during pre-chemotherapy counselling sessions with reproductive-age cancer patients, in regard to the expected decrease in their future reproductive potential. It is also important from the perspective that the efficacy of in vitro follicular culture can be assumed using cryopreserved ovarian tissue collected after the initiation of chemotherapy.
This study aimed to investigate the concentrations at which cisplatin and paclitaxel affect the in vitro development of ovarian follicles. The effects of each agent alone or their combination on the follicular growth pattern were studied. In addition, whether these effects differentially affect the oocytes and surrounding granulosa cells was also evaluated.
Material and methods
Ethics
All of the experimental procedures in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital (#15-0016-S1A0).
In vitro follicular development
Thirteen-day-old C57BL/6 mice were sacrificed [6–10], and each ovary was isolated as previously reported. Collected ovaries were dissociated mechanically and single follicles larger than 100 µm diameter were selected and grown in a single droplet of each media. Half of the volume of medium was changed every 2 days until day 13. On day 13, human chorionic gonadotropin (hCG, Ovidrel®, Merck Serono) at 100 mIU and epidermal growth factor (EGF, Invitrogen) at 10 ng/ml were added to induce the extrusion of cumulus-oocyte complex (COC). Grown follicle was classified as healthy and well-grown if the follicles remained normal in structure, with close contact between oocyte and the surrounding granulosa cells (G-cells), and as degenerated follicles if the oocyte and G-cells were not visible under microscopic observation.
Measurement of follicular diameter, survival, ovulation, and maturation rates
The diameters of growing follicles were measured between the poles of extreme theca cells using i-solution program (i-solution, Daejeon, Korea), and the average of each follicle was calculated. Survival of each follicle was calculated by counting the follicles expanded in vitro after seeding. After 36 h of hCG and EGF treatments, the number of ovulated COCs containing healthy oocyte at the stage of metaphase I (MI) or metaphase II (MII) was observed.
Preparation of culture medium and treatment with chemotherapeutic agents
Preparation of culture media was performed as reported previously. Briefly, MEM alpha (Invitrogen, CA, USA) was used as basal medium. Medium was supplemented with 5% foetal bovine serum (FBS, HyClone, Logan, UT, USA), 10 mM penicillin-streptomycin (Invitrogen), and 1 mM insulin-transferrin-selenium (ITS, Invitrogen). In addition, culture media was supplemented with 200 mIU/ml of recombinant human FSH (Gonal-F®, Merck Serono) and 100 mIU/ml of LH (Luveris®, Merck Serono). Finally, 25 µl of media was seeded as droplets on a culture dish and covered with mineral oil (Sigma-Aldrich, St. Louis, MO, USA).
Stock solutions of cisplatin (Sigma-Aldrich) were prepared using 0.5 M NaCl (Sigma-Aldrich), and Paclitaxel (Sigma-Aldrich) was prepared at a concentration of 1 × 10–3 M. Final concentrations in the culture medium were 10–8, 10–9, and 10–10 M. In the experimental group, follicles were cultured in each group of medium containing cisplatin (C), paclitaxel (P), and cisplatin with paclitaxel (C + P).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from ovarian follicles using Trizol (Invitrogen). cDNA was synthesised from 0.5 µg of total RNA and using Accute premix (Bioneer, Daejeon, Korea). Each specific primer set was added to the cDNA, and the reactions were amplified under the following conditions: incubation for 10 min at 95°C, denaturation for 20 s at 95°C, annealing for 50 s at 58°C and 30 s for 72°C for extension. All the reactions were carried out in triplicate and the expression of Ct was calculated based on the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression (Table I).
Table I
Primer sequences used for qRT-PCR
Immunostaining analysis
Follicles of each group were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA, Sigma-Aldrich) for 20 min at room temperature. They were incubated with bovine serum albumin (BSA, Sigma-Aldrich) blocking solution at 4°C overnight.
Primary antibody, rabbit anti-mouse X-linked inhibitor of apoptosis protein (XIAP) (Abcam, Cambridge, UK), was treated at 4°C overnight and washed three times with PBST (PBS containing Triton X-100, Sigma-Aldrich). Secondary antibody, goat anti-rabbit IgG 488 (Molecular Probes, CA, USA) was added and incubated for 30 min at 37°C and washed three times with PBST. For the staining of the nucleus, 10 µM of DAPI was added and samples were observed under a fluorescence microscope (EVOS-FL, Life Technologies, USA).
Transmission electron microscope (TEM) analysis
Dissected follicles were washed with PBS and then fixed with 2.5% glutaraldehyde (Sigma-Aldrich). Samples were fixed with 2% aqueous OsO4 (Sigma-Aldrich) and serially dehydrated. Then, dehydrated samples were embedded, sectioned, and observed using JE-1000 (Japan).
Results
Determination of treated chemo-reagent concentrations
The concentration range of each reagent between 10–8 M and 10–10 M was tested. To determine the treatment concentrations, those were selected that led to the apoptosis of in vitro grown follicles, not a lethal dose of cell death during in vitro development of isolated, pre-antral follicles. 10–10 M of each reagent was decided as the treatment concentration (Figure 1).
Effects of chemo-reagents on in vitro follicle development and oocyte maturation
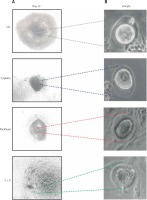
The control group showed in vitro matured and ovulated normal oocytes after hCG treatment. To evaluate the effects of chemo-reagents, 10–10 M of cisplatin and paclitaxel, alone or in combination, were treated to the in vitro growing day 5 follicles for 5 days. The expansion degree of each follicle of treated groups was significantly decreased compared to controls and the follicles gradually degenerated. Ovulation was not observed in all the treated groups (Figure 2 A, Supplementary Figure S1).
Figure 2
In vitro development of murine follicles in the presence of cisplatin (C) and paclitaxel (P). A – Morphological observation of in vitro grown follicles in the presence or not of C, P, C + P, B – survival rate of follicles grown in the presence of chemo-reagents. The survival rate is calculated based on the number of expanded follicles divided by the number of seeded follicles, magnification: 40.
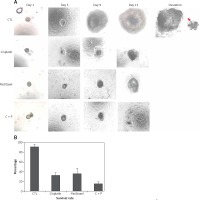
The survival rates were further decreased in the combination group compared to single agent groups (Figure 2 B). Most of the treated groups showed abnormal morphology such as shrinkage of follicles and extrusion of oocytes (Figure 3), while the control group showed ovulated mature oocytes. Degeneration of follicles and oocytes was more remarkable in the combined group than in single agent groups.
Expression of apoptosis- and development-related genes and localisation of XIAP
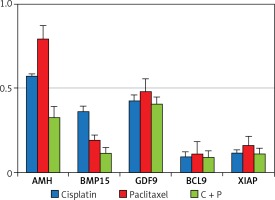
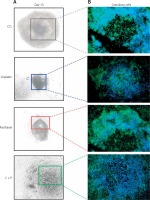
The expression of anti-apoptosis genes in the G-cells, Bcl2, and XIAP, was significantly down-regulated by the treatment with chemo-reagents, either alone or in combination. The expression of oocyte development-related genes, growth differentiation factor 9 (GDF9), and bone morphogenetic protein 15 (BMP15) was also down-regulated. Combined treatment culminated in further suppression of the expression of all evaluated genes (Figure 4). The expression of XIAP protein was observed in the cytoplasm of G-cells. Its expression was decreased in all treated groups (Figure 5).
Ultra-structural analysis of chemo-reagent treated follicles
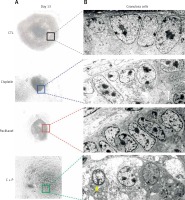
When the ultra-structure of G-cells was analysed, control groups showed evenly distributed cells around oocyte. In contrast, the treated groups showed various sizes, morphologies, and uneven distributions of G-cells. They had the accumulation of metabolic waste such as lipid droplets and degenerated or non-expanded mitochondria (Figure 6).
Figure 6
Microstructure of chemo-reagents treated follicles. A – Microscopic image of in vitro matured, chemo-reagent treated follicles, magnification: 100. B – Ultra-structural analysis of G-cell using transmission electron microscope, magnification: 5000. The yellow arrow indicates precipitated metabolic droplet
Discussion
This study showed differential effects of cisplatin and paclitaxel on in vitro follicular growth, in terms of oocyte and follicular cells. The observed effects were significantly different between oocytes and G-cells regarding their gene expression and morphological alteration. To our knowledge, this is among the first studies to evaluate the effects of cisplatin and paclitaxel on in vitro developing murine follicles using a previously settled system by our group [6, 7, 11].
Along with the increased survival after chemotherapy of cancer patients, fertility preservation in female patients became a rising issue in the gynaecological oncology field [12]. Chemotherapy in female cancer patients leads to the death of follicles in the ovary and often culminates in infertility. To preserve the fertility of women in reproductive age, prudent choice of chemo-agents and the cryopreservation of ovarian tissue before treatment can be important issues.
Cisplatin and paclitaxel are widely used in gynaecological cancer patients [1, 4], and numerous studies have been performed to elucidate their effects on ovarian function [13]. To date, their toxic effects on the ovarian reserve were demonstrated by assessing the damage to ovarian tissue and diminished residual follicles, via experiments using ovarian tissue or whole ovaries [14, 15]. Recent in vitro studies using ovarian tissue culture found that, in a dose-dependent manner, taxane drugs caused the primary follicle depletion and that granulosa cells were the first cells to be affected [16].
To establish the strategy for fertility preservation, it is important to evaluate the chemo-drugs’ effects on developing follicles [11]. The cells of developing follicles are proliferating actively, and they can be targets of chemo-agents [17]. It may be expected that chemo-drugs affect the G-cells more signifiacntly than non-dividing oocytes. And chemo-reagents could affect developing stages of pre-antral and antral follicles more negatively (Figure 1).
Evidence from a previous report suggested that the treatment of taxanes can induce apoptosis [18], while another study showed that taxanes only had minimally toxic effects on the ovarian function [19]. In other previous studies, paclitaxel treatment suppressed follicle growth and antral formation [20].
Taxanes are usually administered in combination with other chemotherapeutic agents, and, in this case, their own toxic effects against ovarian function are difficult to determine [21, 22]. Paclitaxel is known to delay oocyte maturation and to induce oocytes aneuploidy [23]. Other in vivo studies using high-dose paclitaxel showed that paclitaxel treatment induced primordial follicle loss [24, 25]. A previous report on the treatment of paclitaxel showed the increased apoptosis of G-cells in developing follicles [20]. It seems that paclitaxel is more toxic to earlier stage follicles in vitro and in vivo [26]. However, the analyses of their effects on developing follicles is still inadequate due to ethical limitations, restricted supply of human follicles, and the absence of suitable models of the in vitro follicular maturation system.
Bcl-2 acts as an anti-apoptotic regulator, and this gene is expressed in mammalian oocytes and G-cells [27]. In the mammalian ovary, the levels of anti-apoptotic and pro-apoptotic gene expression are known to be low in healthy follicles and increased in atretic follicles [28, 29].
The expression of all tested genes was down-regulated, and the affected degree differed among the treated groups. AMH and GDF9 demonstrated higher expression in the paclitaxel-treated group (Figure 4). GDF-9, expressed in oocytes, is known to be a paracrine regulator of growth and differentiator of ovarian somatic cells [30]. The relatively high expression of GDF-9 in the present study may have been caused by the generation of reactive oxygen species (ROS) disrupting the membrane when paclitaxel is treated [31].
In the present study, combined treatment significantly increased the apoptosis of growing follicles, and the expression of Bcl-2 was decreased. These results are correlated with expression of XIAP, another anti-apoptotic factor related to the development of follicles. XIAP, a known inhibitor of apoptosis protein 3 (IAP3), is a protein that stops apoptotic cell death. XIAP is expressed in all the stages of follicle development and promotes survival of follicles by inhibiting caspase-3, -7, and -9 [32, 33]. Overexpression of XIAP significantly enhances the survival of pre-antral follicles, and thus XIAP could be used as a pre- and post-chemo protective agent due to its distinct role in regulating follicle atresia.
The regulation of follicle atresia is an important issue in fertility preservation. Currently, a number of strategies including gonadotropin-releasing hormone (GnRH) agonist are used for the protection of ovarian reserve [34]. It interferes with the hypothalamic-pituitary-gonadal axis and blocks ovarian function by suppressing gonadotropin levels to pubertal levels. However, its effect is not satisfactory for the preservation of ovarian function [35]. Therefore, the pre- and post-chemo rescuing of follicles using XIAP could be a candidate strategy for maintaining ovarian reserve.
In conclusion, we demonstrated the effects of cisplatin and paclitaxel on in vitro follicular growth. Their effects were differentially observed between oocytes and G-cells regarding the gene expression and morphological variation. Further investigations are necessary to evaluate the potential efficacy of XIAP as an anti-apoptotic regulator when used before chemotherapy. The potential use of stem cells extracted from ovaries should also be evaluated in regard to their supportive roles during in vitro murine and primate follicle culture setting of this study.