Introduction
As of December 2020, almost 80 million people have been infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 COVID-19 [1, 2]. This equates to more than 1% of the world population [3]. The total number of COVID-19-related deaths has now reached more than 1.7 million, which translates to a rate of 2.2% of the cases. In the face of this, the last several weeks have seen the rollout of new vaccines for protection against COVID-19, which have been produced in record time due to an unprecedented worldwide effort [4–6]. Although massive worldwide networks have already been put in place to distribute these vaccines across the globe, we are still several months away from seeing a drop in daily COVID-19 case numbers, hospitalisations, and mortality. Therefore, there is still an urgent need to control the disease spread by testing of new drugs and compounds and by repurposing existing therapeutics [7, 8].
Epidemiological studies have shown that age and preexisting medical conditions such as hypertension, cardiopulmonary diseases, diabetes and obesity are associated with a more severe disease course and higher mortality in patients hospitalized with COVID-19 disease [9–13]. However, it is still not known why some of these individuals experience a more severe form of the diseasewhile others in the same high-risk groups only show mild symptoms or are asymptomatic. One possible explanation is due to the protective effects of medications routinely used to treat these conditions, although this has been debated [14, 15].
Statins are inexpensive, easily available, and are already in widespread use for treatment of cardiovascular disorders, which has created considerable interest in further exploration of their repurposing as a new treatment for managing the severity of COVID-19 infections [16–21]. Statins are known to reduce the risk of major cardiovascular events and to counteract inflammation, the immune response, and oxidative stress damage [22–25], which may reduce the risk of a more severe disease course and improve outcomes in COVID-19 patients. However, it should be emphasised that there is still a lack of clinical consensus on the efficacy of statins in improving disease outcomes in COVID-19 patients, particularly with respect to whether or not the drug has been administered before or after diagnosis.
To address these points, we have carried out a systematic review and meta-analysis to investigate the effect of statins on COVID-19 disease outcomes. In particular, we focused on the rate of intensive care unit (ICU) admission, the need for mechanical ventilation, and mortality. It was also of special interest to determine whether or not there was a difference in these outcomes in patients who had received statins before or after their COVID-19 diagnosis.
Material and methods
Search strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Ethical approval was obtained from the research ethics committee of Baqiyatallah University of Medical Sciences with the ethics code of IR.BMSU.REC.1399.009 in 2020-03-26. The searches were conducted in the Web of Science, PubMed, Scopus, and ProQuest databases for eligible articles up to November 2, 2020, with no restrictions on language or publication date. The reference lists of articles were also reviewed using forward and backward citation tracking to identify other eligible documents. The population, intervention, comparison, and outcome (PICO) criteria were formulated as follows:
population: patients infected with SARS-CoV-2 confirmed by reverse transcriptase polymerase chain reaction (RT-PCR);
intervention: statin therapy;
comparison: patients infected with SARS-CoV-2 who did not use statins;
outcomes: intensive care unit (ICU) admission, tracheal intubation, and all-cause mortality.
The overall aim was to determine whether or not a relationship exists between statin therapy and improvement of outcomes in patients infected with SARS-CoV-2. The following is an example of the PubMed title and abstract search strategy:
novel coronavirus OR novel coronavirus 2019 OR 2019 novel coronavirus OR 2019 nCoV OR Wuhan coronavirus OR Wuhan pneumonia OR covid-19 OR 2019-nCoV OR SARS-CoV-2 OR coronavirus 2019 OR 2019-nCoV
AND
statin OR HMGCoA reductase inhibitor OR lovastatin OR fluvastatin OR pravastatin OR rosuvastatin OR pitavastatin OR atorvastatin OR simvastatin OR cerivastatin OR lipitor OR lescol OR lecol AND xl OR mevacor OR altoprev OR pravachol OR crestor OR zocor OR livalo.
To ensure comprehensive searches of all articles related to the effect of statins on outcomes, we followed a search strategy without considering specific outcomes. After finding all relevant articles, we concluded that only 3 outcomes were amenable to meta-analysis due to sufficient numbers of studies including the parameters in the literature: 1) ICU admission; 2) tracheal intubation; and 3) all-cause mortality. Therefore, the study continued with a focus on these 3 outcomes.
Inclusion and exclusion criteria
The inclusion criteria were as follows:
observational studies (case-control studies and cohort studies) and randomised clinical trials evaluating the impact of statins on patients with COVID-19 in either prospective or retrospective formats;
outcome measures that included all-cause mortality, ICU admission, and tracheal intubation.
The exclusion criteria were as follows:
clinical case reports, literature reviews, animal studies, and studies involving in vitro experiments;
studies not including statin non-users.
Study selection
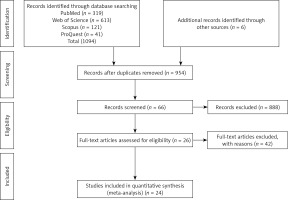
We performed a 4-step process carried out in parallel by 2 authors (AHS and AVA) to determine eligible criteria for inclusion. First, we searched the PubMed, Web of Science, Scopus, and ProQuest databases and identified 1094 potentially relevant studies. Six additional studies were identified via Internet searches, bringing the total number of articles to 1100. Next, we exported the identified records into the EndNote X9 software, reviewed the full list, and excluded duplicate studies, leaving 954 records. The titles and abstracts of these articles were then screened, and relevant studies selected for full text evaluation. The full texts of the 66 remaining studies were assessed for eligibility, leaving 26 studies. Finally, 24 studies that met all inclusion and exclusion criteria were selected for the analysis (Figure 1).
Data extraction
Data extraction was completed independently by 2 researchers (FHB and AVA), and the data form was populated to include the following: authors name, sample size, subjects, reported rate, setting, study design, result, and conclusions (the year was not included because all studies were from 2020). The main outcomes were also summarised. Discrepancies in extracted data were resolved by discussion between authors. Remaining disagreements were resolved by a third reviewer. The extracted data are displayed in Table I.
Table I
Extracted data of studies. All studies were conducted in 2020
| Author | Statin | Sample | Setting | Study design | Result | Conclusion | |
|---|---|---|---|---|---|---|---|
| User | Non-user | ||||||
| ICU admission: | |||||||
| Masana et al. [55] | 581 | 1576 | Patients admitted to their hospitals because of SARS-CoV-2 infection | Members of the Lipids and Arteriosclerosis Units Net (XULA) of Catalonia (Spain) | Retrospective observational | N/A | N/A |
| 103 (17.7%) | 233 (14.8%) | ||||||
| Zhang et al. [52] | 1219 | 12762 | Patients with COVID-19 | Hubei Province, China | Retrospective | aHR = 0.69, CI: 0.56–0.85, p = 0.001 | Cox model analysis showed statin use associated with lower prevalence ICU admission |
| N/A | N/A | ||||||
| Song et al. [56] | 123 | 126 | Patients with COVID-19 | “Lifespan” healthcare system hospitals | Retrospective cohort | OR = 0.90, CI: 0.49–1.67, p = 0.756 | No significant associations between statin use and hospital death or ICU admission |
| N/A | N/A | ||||||
| Argenziano et al. [57] | 325 | 525 | Patients with laboratory confirmed covid-19 infection | New York-Presbyterian/Columbia University Irving Medical Center, a quaternary care academic medical center | Retrospective case series | OR = 1.07, CI: 0.79–1.46 | N/A |
| 93 (28.6%) | 143 (27.2%) | ||||||
| De Spiegeleer et al. [58] | 31 | 123 | Residents at two elderly care homes with COVID-19 diagnosis | One of two Belgian nursing homes | Retrospective multi-centre cohort | OR = 0.75, CI: 0.24–1.87 | Statin use showed non-significant benefits |
| 6 (19.3%) | 31 (25.2%) | ||||||
| Yan et al. [47] | N/A | N/A | Confirmed Covid-19 diagnosis | Hospitals in Zhejiang province, China | Case-control | OR = 0.98, CI: 0.32–2.99, p = 0.973 | N/A |
| N/A | NA | ||||||
| Dreher et al. [59] | 18 | 32 | COVID-19 patients with and without acute respiratory distress syndrome [ARDS) | Aachen University Hospital | Retrospective cohort | OR = 1.13, CI: 0.36–3.60 | N/A |
| 9 (50.0%) | 15 (46.8%) | ||||||
| Tan et al. [60] | 40 | 509 | 717 patients admitted | Tertiary centre in Singapore for COVID-19 infection | Retrospective cohort | ATET Coeff: –0.12, CI: –0.23–0.01, p = 0.028 | Statin use independently associated with lower requirement for ICU admission |
| 1 (2.5%) | N/A | ||||||
| ICU admission: | |||||||
| Daniels et al. [61] | 46 | 124 | Patients hospitalised for treatment of COVID-19 | University of California San Diego Health (UCSDH), ascertained by data capture within system-wide electronic health record (EHR) system (Epic Systems, Verona, WI, USA) | Retrospective cohort | Statin use prior to admission associated with reduced risk of severe COVID-19 (adjusted OR = 0.29, CI: 0.11–0.71, p < 0.01) | In patients hospitalised for COVID-19, use of statin medication prior to admission associated with reduced risk of severe disease |
| 20 (43.4%) | 70 (56.4%) | ||||||
| Vahedian-Azimi et al. [54] | 326 | 525 | Positive for SARS-CoV-2 | Baqiyatallah University of Medical Sciences | Prospective observational | OR = 1.00, CI: 0.58–1.74, p = 0.736 | Statin use not associated with mortality |
| 39 (11.9%) | 243 (46.2%) | ||||||
| Tracheal intubation: | |||||||
| Zhang et al. [52] | 1219 | 12762 | Patients with COVID-19 | Hubei Province, China | Retrospective | aHR = 0.37, CI: 0.26–0.53, p < 0.001 | Cox model analysis showed statin use associated with a lower prevalence of using mechanical ventilation |
| N/A | N/A | ||||||
| Song et al. [56] | 123 | 126 | Patients with COVID-19 | “Lifespan” healthcare system hospitals | Retrospective cohort | Statin use significantly associated with decreased risk for IMVOR: 0.45, CI: 0.20–0.99, p = 0.048 | Data support continued use of statins in patients hospitalised with COVID-19 due to decreased risk for IMV |
| N/A | N/A | ||||||
| Gupta et al. [62] | 648 | 648 | Positive for SARS-CoV-2 | Columbia University Irving Medical Center (CUIMC) and Allen Hospital sites of the New York-Presbyterian Hospital (NYPH) | Retrospective | No significant difference in invasive mechanical ventilation | N/A |
| 130 (20.1%) | 158 (24.4%) | ||||||
| Masana et al. [55] | 581 | 1576 | Patients admitted to hospitals due to SARS-CoV-2 infection | Members of the Lipids and Arteriosclerosis Units Net (XULA) of Catalonia (Spain) | Retrospective observational | N/A | N/A |
| 84 (14.5%) | 191 (12.1%) | ||||||
| Cariou et al. [63] | 112 | 1257 | Patients with diabetes admitted with COVID-19 | 68 French hospitals | Nation-wide observational | OR = 1.13, CI: 0.83–1.53 | Routine statin use not significantly associated with increased risk of tracheal intubation/mechanical ventilation |
| 229 (19.2%) | 248 (19.7%) | ||||||
| Tan et al. [60] | 40 | 509 | Patients admitted for COVID-19 | Tertiary centre in Singapore for COVID-19 infection | Retrospective cohort | ATET Coeff: –0.08, CI: –0.19–0.02, p = 0.114 | No significant differences in intubation |
| 1 (2.5%) | N/A | ||||||
| Peymani et al. [64] | 75 | 75 | Hospitalised COVID-19 patients | Single tertiary hospital in Shiraz, Iran | Retrospective | OR = 0.96, CI: 0.61–2.99, p = 0.942 | Non-significant association between statin use and reduction in mortality in COVID-19 patients |
| N/A | N/A | ||||||
| Mortality | |||||||
| Gupta et al. [62] | 648 | 648 | Positive for SARS-CoV-2 | Columbia University Irving Medical Center (CUIMC) and Allen Hospital sites of the New York-Presbyterian Hospital (NYPH) | Retrospective | Univariate – OR = 0.69, CI: 0.56–0.85 Multivariate adjusted – OR = 0.49, CI: 0.38–0.63 | Antecedent statin use associated with significantly lower rates of in-hospital mortality within 30 days |
| 112 (17.2%) | 201 (31.0%) | ||||||
| Masana et al. [55] | 581 | 581 | Patients admitted to hospitals due to SARS-CoV-2 infection | Members of the Lipids and Arteriosclerosis Units Net (XULA) of Catalonia (Spain) | Retrospective observational | Significant difference in mortality rate between groups – HR = 0.58, CI: 0.39–0.89, p = 0.01 | A lower SARS-CoV-2 infection-related mortality observed in patients treated with statin therapy prior to hospitalization |
| 115 (19.8%) | 148 (25.4%) | ||||||
| Zhang et al. [52] | 1219 | 12762 | Patients with COVID-19 | Hubei Province, China | Retrospective | Individuals with statin therapy had a lower crude 28-day mortality (Incidence rate ratios (IRR): 0.78, CI: 0.61–1.00, p = 0.046) | Statin use in hospitalized COVID-19 patients associated with lower risk of all-cause mortality and favorable recovery profile |
| 0.21%* | 0.27%* | ||||||
| Rossi et al. [65] | 42 | 29 | Patients with pre-existing chronic cardiovascular disease, with COVID-19 | N/A | Observational | Mortality rates of patients taking statins was 21.4% (9/42), and 34.5% (10/29) in those not taking statins (p < 0.05) | Statin use significantly reduced risk of mortality in COVID-19 patients |
| 9 (21.4%) | 10 (34.5%) | ||||||
| Cariou et al. [63] | 1192 | 1257 | Patients with diabetes admitted with COVID-19 | 68 French hospitals | Nation-wide observational | Mortality rates significantly higher in statin users in 28 days (23.9% vs. 18.2%, p < 0.001), OR = 1.46, CI: 1.08–1.95 | Routine statin treatment significantly associated with increased mortality in T2DM patients hospitalized for COVID-19 |
| 285 (23.9%) | 229 (18.2%) | ||||||
| Saeed et al. [50] | 983 | 1283 | Patients with diabetes mellitus hospitalized with COVID-19 | Montefiore Medical Center, Bronx, New York | Observational retrospective | Patient with diabetes on statins had lower cumulative in-hospital mortality (24% vs. 39%, p < 0.01). HR = 0.51, CI: 0.43–0.61, p < 0.001 | Statin use associated with reduced in-hospital mortality from COVID-19 in patients with diabetes |
| 236 (24.0%) | 500 (39.0%) | ||||||
| Saeed et al. [50] | 372 | 1614 | Patients without diabetes mellitus hospitalized with COVID-19 | Montefiore Medical Center in Bronx, New York | Observational retrospective | No difference noted in patients without diabetes (20% vs. 21%, p = 0.82) | Statin use associated with reduced in-hospital mortality from COVID-19 in patients with diabetes |
| 74 (20.0%) | 339 (21.0%) | ||||||
| Song et al. [56] | 123 | 126 | Patients with COVID-19 | “Lifespan” healthcare system hospitals | Retrospective cohort | No significant associations between statin use and in hospital death OR = 0.88, CI: 0.37–2.08, p = 0.781 | No significant associations between statin use and hospital death |
| N/A | N/A | ||||||
| De Spiegeleer et al. [58] | 31 | 123 | Residents at 2 elderly care homes with COVID-19 diagnosis | 1 of 2 Belgian nursing homes | Retrospective multi-centre cohort | Considering death as serious outcome, the effects sizes, OR = 0.61, CI: 0.15–1.71, p = 0.380 | Statins not statistically significant associated with death from COVID-19 in elderly adults in nursing homes |
| N/A | N/A | ||||||
| Rodriguez-Nava et al. [53] | 47 | 40 | Laboratory-confirmed COVID-19 | Community hospital intensive care unit (ICU) located in Evanston, IL | Retrospective cohort | Multivariable Cox PH regression model showed atorvastatin non-users had 73% chance of faster progression to death compared with users. HR = 0.38, CI: 0.18–0.77, p = 0.008 | Slower progression to death associated with atorvastatin use in patients with COVID-19 admitted to ICU |
| 23 (49.0%) | 25 (63.0%) | ||||||
| Zenga et al. [66] | 38 | 993 | COVID-19 inpatients | Tongji Hospital, Tongji Medical College of HUST (Wuhan, China) | Retrospective cohort | OR = 0.79, CI = 0.3–2.05 | N/A |
| 5 (13.1%) | 160 (16.1%) | ||||||
| Nguyen et al. [67] | 90 | 266 | African American Population with COVID-19 | University of Chicago Medical Center (UCMC), serving south metropolitan Chicago | Retrospective observational | OR = 0.81, CI: 0.39–1.72 | N/A |
| 10 (11.1%) | 35 (13.1%) | ||||||
| Wang et al. [34] | 24 | 12 | multiple myeloma patients with COVID-19 | Mount Sinai Hospital | Retrospective | Statin use significantly associated with mortality. OR = 6.21, CI: 1.37–39.77, p = 0.012 | N/A |
| 11 (45.8%) | 3 (25.0%) | ||||||
| Grasselli et al. [46] | N/A | N/A | Patients admitted to ICUs in Lombardy with suspected SARS-CoV-2 infection | One of the Network ICUs, Milan | Retrospective, observational study | Statins associated with higher mortality in univariate analysis. HR = 0.98, CI: 0.81–1.2, p = 0.87 | Long-term treatment with statins, before ICU admission associated with higher mortality un-adjusted analysis only. Multivariate analysis did not confirm association between any home therapies and increased mortality |
| N/A | N/A | ||||||
| Ayed et al. [68] | 10 | 93 | Intensive care unit intensive care unit (ICU)-admitted COVID-19 patients | Jaber Al-Ahmad Al Sabah Hospital, Kuwait | Retrospective cohort | OR = 0.49, CI: 0.11–2.08 | N/A |
| 4 (40.0%) | 43 (46.2%) | ||||||
| Tan et al. [60] | 40 | 509 | 717 patients admitted | Tertiary centre in Singapore for COVID-19 infection | Retrospective cohort | ATET Coeff: –0.04, CI: –0.16–0.08, p = 0.488 | No significant differences in mortality |
| 2 (5.0%) | N/A | ||||||
| Peymani et al. [64] | 75 | 75 | Hospitalised COVID-19 patients | Single tertiary hospital, Shiraz, Iran | Retrospective | HR = 0.76, CI: 0.16–3.72, p = 0.735 | Non-significant association between statin use and reduction in mortality in patients with COVID19 |
| N/A | N/A | ||||||
| Nicholson et al. [69] | 511 | 531 | 1042 people with COVID-19 symptoms admitted | Mass General Brigham Hospitals | Retrospective cohort | OR = 0.50, CI: 0.27–0.93, p = 0.027 | Chronic statin use associated with reduced in-hospital mortality |
| N/A | N/A | ||||||
| Lala et al. [70] | 984 | 1752 | Hospitalized COVID-19 positive patients | 1 of 5 Mount Sinai Health System hospitals in New York City | Multihospital retrospective cohort | HR = 0.57, CI: 0.47–0.69, p < 0.001 | Statin use associated with improved survival |
| N/A | N/A | ||||||
| Krishnan et al. [35] | 81 | 71 | Consecutive patients requiring mechanical ventilation from March 10 to April 15 | St. Joseph Mercy Oakland Hospital | Retrospective observational | OR = 2.44, CI: 1.23–4.76, p = 0.0080 | Statin use associated with increased mortality |
| N/A | N/A | ||||||
| Vahedian-Azimi et al. [54] | 326 | 525 | Positive for SARS-CoV-2 | Baqiyatallah University of Medical Sciences | Prospective observational | OR = 0.18, CI: 0.06–0.49, p = 0.0001 | Statin use associated with decreased mortality |
| 8 (2.5%) | 282 (53.7%) | ||||||
Quality assessment
The methodological quality assessment of studies was performed independently by 2 authors (FHB and AVA) using the Newcastle-Ottawa Scale (NOS) for cohort studies, and disagreements were resolved by discussion between the authors. The NOS scale was developed to assess the quality of nonrandomised studies with respect to design, content, and ease of use in the interpretation of results of meta-analyses. Using this scale, each study was assessed according to 3 broad perspectives: 1) selection of the study groups; 2) comparability of the groups; and 3) the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively [26]. A rating system of 0–3 stars per category was used to indicate quality, giving a range of 0–9 total stars per study [27]. Disagreements about inclusion criteria, data extraction, and quality assessment were resolved by consensus. The quality assessment of the studies is shown in Table II.
Table II
Quality assessment of studies by Newcastle-Ottawa scale (NOS)
| Studies | Selection of cohorts | Comparability of cohorts | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration outcome of interest does not present at start of study | Comparability on the basis design or analysis | Assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Gupta et al. [62] | * | * | * | ** | * | * | 7 | ||
| Masana et al. [55] | * | * | * | ** | * | 6 | |||
| Zhang et al. [52] | * | * | * | * | ** | * | * | * | 9 |
| Rossi et al. [65] | * | * | * | * | ** | 6 | |||
| Cariou et al. [63] | * | * | * | ** | * | * | * | 8 | |
| Saeed et al. [50] | * | * | * | * | ** | * | * | * | 9 |
| Song et al. [56] | * | * | * | * | ** | * | * | 8 | |
| De Spiegeleer et al. [58] | * | * | * | * | ** | * | 7 | ||
| Rodriguez-Nava et al. [53] | * | * | * | ** | 5 | ||||
| Zenga et al. [66] | * | * | * | ** | * | * | 7 | ||
| Nguyen et al. [67] | * | * | * | * | ** | * | * | 8 | |
| Wang et al. [34] | * | * | * | ** | * | 6 | |||
| Grasselli et al. [46] | * | * | * | * | ** | * | * | * | 9 |
| Yan et al. [47] | * | * | * | ** | * | * | 7 | ||
| Ayed et al. [68] | * | * | * | * | ** | * | * | 8 | |
| Tan et al. [60] | * | * | * | ** | * | * | 7 | ||
| Peymani et al. [64] | * | * | * | * | * | * | 7 | ||
| Nicholson et al. [69] | * | * | * | * | * | * | * | * | 9 |
| Lala et al. [70] | * | * | * | * | * | * | * | * | 9 |
| Krishnan et al. [35] | * | * | * | ** | * | * | 7 | ||
| Argenziano et al. [57] | * | * | * | * | ** | * | * | * | 9 |
| Daniels et al. [61] | * | * | * | * | ** | * | * | * | 9 |
| Dreher et al. [59] | * | * | * | * | ** | * | * | 8 | |
| Vahedian-Azimi et al. [54] | * | * | * | * | ** | * | * | * | 9 |
Statistical analysis
All analyses were conducted using STATA16 (StataCorp, College Station, Texas, USA). The reporting of the study was adapted based on the PRISMA statement [28]. Data extraction for the main outcomes was conducted and random effects meta-analyses were carried out using the restricted maximum likelihood method [29]. The random effects model was applied due to the possible existence of other unknown, unregistered, or unpublished studies, which we could not access. Between-study heterogeneity was evaluated using Cochran Q test, tau-squared (t2), H-squared (H2), and I-squared (I2) statistics. The significance of the results of the tests and values higher than 75% for I2 were considered as having substantial heterogeneity, while H2 = 1 was taken to represent perfect homogeneity among the studies [30]. To assess the publication bias, funnel plots, regression-based Egger’s [31], and nonparametric rank correlation-based Begg’s [32] tests were used to test for the presence of small-study effects often associated with publication bias. A funnel plot is a scatter plot of study-specific effect sizes on the x axis against standard errors on the y axis. In the absence of small-study effects, the plot should look symmetrical. A nonparametric trim-and-fill method of accounting for publication bias was conducted, and the modified effect size was estimated after adjusting for publication bias. This estimates the number of studies potentially missing from a meta-analysis because of publication bias, imputes these studies, and computes the overall effect-size estimate using the observed and imputed studies. This can also be used to generate a funnel plot, in which omitted studies are imputed [33]. Common effect sizes were calculated as an odds ratio (OR) with 95% confidence interval (CI) for each main outcome, and the results were presented using forest plots. The individual and the overall effect sizes, CIs, heterogeneity statistics, and significance tests for effect size were reported. Additionally, predetermined subgroup analyses were conducted according to the conditions of either in- or pre-hospital use of the statin treatments.
Results
ICU admission
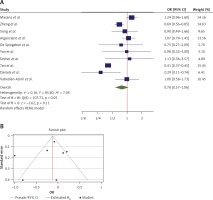
The OR from 10 studies was 0.78 (95% CI: 0.58–1.06, p = 0.11), with significant heterogeneity between studies (τ2 = 0.16, I2 = 85.80%, H2 = 7.04, Q(df=9) = 107.72, pQ < 0.001) (Figure 2 A). Assessment for bias by Egger’s (p = 0.815) and Begg’s (p = 0.142) tests showed no significant small-study effects. Further visual inspection of the funnel plot suggested a slight degree of publication bias (Figure 2 B). Extending the results of the analyses for the nonparametric trim-and-fill method of accounting for publication bias showed that there should be 2 additional studies for inclusion (Figure 2 C). A re-estimation of the overall OR after these studies was included, which resulted in an OR = 0.70 (95% CI: 0.52–0.94).
Figure 2
A – Forest plot of individual effect sizes within each study for ICU admission outcome. The overall effect is shown by the green symbol. B – Funnel plot showing publication bias for ICU admission outcome. C – Extending the results using the nonparametric trim and fill method to account for publication bias. Two additional studies were included in this analysis (shown in red)
Tracheal intubation
The OR from 7 studies was 0.79 (95% CI: 0.57–1.11, p = 0.18) based on the random effects model analysis, with significant heterogeneity between studies (τ2 = 0.16, I2 = 88.99%, H2 = 9.08, Q(df=6) = 34.9, pQ < 0.001) (Figure 3 A). Assessment for bias by Egger’s (p = 0.447) and Begg’s (p = 0.115) tests showed no significant small-study effects, and visual inspection of the funnel plot suggested no publication bias (Figure 3 B). Therefore, we did not extend the results of the analyses for nonparametric trim and fill method of accounting for publication bias in this case.
Death
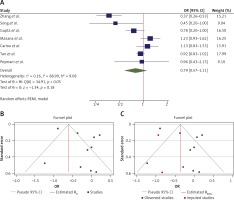
The OR from 21 studies which assessed the effect of statins on all-cause mortality was 0.70 (95% CI: 0.55–0.88, p < 0.01) based on a random effects model, with significant heterogeneity between studies (τ2 = 0.18, I2 = 82.55%, H2 = 5.73, Q(df = 20) = 97.04, pQ < 0.001). Further investigation detected 2 outliers with a large effect size [34, 35]. We removed these from further analyses for this outcome, which resulted in a slight reduction in effect size (OR = 0.64, 95% CI: 0.52–0.79, p < 0.01); although heterogeneity was reduced, it still remained significant (τ2 = 0.11, I2 = 76.50%, H2 = 4.23, Q(df=18) = 76.5, pQ < 0.001) (Figure 4 A). Assessment for bias by Egger’s (p = 0.903) and Begg’s (p = 0.349) tests showed no significant small-study effects, and visual inspection of the funnel plot suggested no publication bias (Figure 4 B).
Figure 4
A – Forest plot showing the effect across the studies assessing pre-hospital (n = 18) and in-hospital (n = 3) use of statins for the death outcome (overall for each subgroup shown as red symbols). The overall effect is shown by the green symbol. B – Funnel plot showing the publication bias for all 21 studies which assessed the death outcome

Subgroup analysis
The forest plot of individual ORs of the predetermined subgroup analyses indicated a significant effect in the pre-hospital use (OR = 0.70, 95% CI: 0.56–0.86, n = 18) and the in-hospital use (OR = 0.40, 95% CI: 0.22–0.73, n = 3) subgroups, with a non-significant difference between the subgroups (Q(df = 1) = 2.86, pQ = 0.09) (Figure 5 C). Additionally, although the heterogeneity was reduced slightly in the 2 subgroups, the amount of reduction was not considerable and remained significant in the pre-hospital group (p < 0.05). The heterogeneity was not significant in the in-hospital group (p = 0.08), which may be due to the smaller number of studies in this subgroup and thus a lower power of the heterogeneity test.
Figure 5
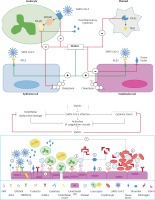
(Upper figure) Proposed mechanisms for the beneficial effects of statins in SARS-CoV-2 infection. Cellular pathways altered by statins during the course of infection in COVID-19 patients. Surface expression of ACE2 in epithelial and endothelial cells is enhanced by statins. Statins also mitigate the MYD88–NF-κB pathway and the ensuing proinflammatory cytokine response following interaction of SARS-CoV-2 with toll-like receptors (TLRs). The anti-thrombotic effects of statins are mediated by suppressive effects on tissue factor, and cytosolic phospholipase A2 (cPLA2)-induced thromboxane A2 (TXA2) synthesis and inactivated endothelial cells and platelets, respectively. Statins have also been suggested to decrease plasma membrane cholesterol content in the host cells, which can interfere with the replication of virus particles. (Lower figure) Proposed mechanisms for the anti-thrombotic effects of statins in SARS-CoV-2 infection SARS-CoV-2 induces activation of endothelial cells and the release of pro-inflammatory cytokines, which can lead to:1) over-expression of adhesion molecules (e.g. P-selectins, intercellular adhesion molecule 1 (ICAM-1), von Willebrand factor, αvβ3) and further release of proinflammatory cytokines; 2) increased recruitment of leukocytes and platelets; 3) activated endothelial cells also express tissue factor, which activates factor VII, factor Xa, and thrombin formation; 4) fibrinogen is cleaved into fibrin by thrombin; 5) thrombin is essential for thrombus formation; 6)pro-inflammatory factors can also promote coagulation and accelerate platelet activation and thrombus formation; and 7)statins downregulate proinflammatory cytokine release from endothelial cells and mitigate coagulation cascade. Moreover, statins interfere with SARS-CoV-2 entry intoACE2- expressing endothelial cells and ensuing endothelial activation (reproduced with permission from [18])
Discussion
Overall, this systematic review and meta-analysis shows a significant benefit of statin use in reducing the severity of COVID-19 disease. We found a 22–30% reduction of the risk of ICU admission and a 30% reduction in the risk of death due to COVID-19 disease. In addition, our subgroup analysis showed that in-hospital use of statins was associated with as much as a 60% reduction in mortality while pre-hospital use was associated with a 30% reduction, although the difference between the subgroups did not reach significance. Statin use was not associated with the tracheal intubation outcome.
Statins may help by preventing the development of severe lung injury and acute respiratory distress syndrome (ARDS) by protecting against inflammation via modulation of cytokine over-expression, angiotensin-converting enzyme 2 (ACE2) expression, and the immune response in COVID-19 patients [18, 36, 37]. Statins have been shown to inhibit nuclear factor κB (NF-κB), which mediates inflammatory responses during infections [38, 39]. Statins may also be used to counteract the cytokine storm, which can also occur in severe cases of viral infection such as in COVID-19 cases [40, 41]. These properties support statin continuation for mitigating ARDS and multiorgan failure, which can occur in the acute phases of COVID-19 illness [42]. In addition, statins can exert direct antiviral effects [43]. We recently produced in-silico evidence showing that statins can bind with high affinity to the main Mpro protease, which mediates viral replication and transcription and could potentially exert anti-SARS-CoV-2 activity through inhibition of viral replication [44]. Statins also exert anti-thrombotic actions through modulation of endothelial cell activation and platelet aggregation, and thereby mitigate coagulopathies associated with infection, the cytokine storm, and impending organ failure [18, 19] (Figure 5).
Our findings regarding the reduced deaths in COVID-19 patients using statins are consistent with mechanistic findings on the anti-thrombotic, anti-inflammatory, antiviral, and immunomodulatory actions of statins [18, 43] as well as those of a recent meta-analysis of 6 studies, which found a pooled 30% risk reduction of severe disease or mortality in COVID-19 patients taking statins [45]. However, 2 of the studies within the previous analyses based statin use on historical record with no mention of pre- or in-hospital administration [46, 47]. Differences in the type of statin used may also account for discrepancies across different studies. This possibility was highlighted in a study by Rossi et al., which showed that administration of simvastatin and atorvastatin reduced mortality in COVID-19 patients, whereas those treated with pravastatin and rosuvastatin showed no such difference [48]. In contrast, the CORONADO study found that statin use was associated with an increased risk of death in diabetes patients with COVID-19 [49], although this conflicted with another study, which found that statin application reduced mortality in COVID-19 diabetic patients [50]. These discrepancies may be due to the use of different statins, because the statin type was not listed in the former study and the latter mainly involved the use simvastatin and atorvastatin.
Another possibility for discrepancies in the pre- hospital use of statins with respect to COVID-19 severity could be due to the presence of diseases, such as hypertension, obesity, or lipid disorders, which are significant risk factors for worse outcomes [10–12]. A study carried out in Denmark showed that recent statin use in COVID-19 patients was not associated with an effect on all-cause mortality or severe infection [51]. It could be that the potential benefit of statin-use was offset by the presence of co-morbidities. Although the researchers attempted to adjust for the potential effects of medication differences, they could not exclude a possible impact on the association between statin use and outcomes, or the presence of pre-existing co-morbidities. Here, we have attempted to address this in our subgroup analysis which compared the effects of pre- and in-hospital use of statins on COVID-19 outcomes. However, future studies should also include an assessment of whether or not pre-existing diseases are present.
The findings of this study should be interpreted by considering a number of limitations. First, the study did not allow any assessment of a cause-and-effect relationship and, therefore, only associations are reported. Second, confounding factors cannot be excluded such as the pre-existing or post-diagnosis development of co-morbidities, including insulin resistance, ARDS, and coagulopathies, which may have affected some outcomes. Third, the findings have not been adjusted for concomitant medications. It should also to be emphasised, that based on the data from the included studies, we were not able to include the sub-analyses on the statin preparations and the investigated outcomes. Finally, our finding that in-hospital statin use was associated with decreased death compared to pre-hospital use did not reach significance. However, this is also likely to be due to the fact that there were only 3 studies [52–54] in the in-hospital use group and this was therefore likely to be statistically underpowered.
In conclusion, we showed that statin therapy might be useful in the context of a lower risk of the severe course of the COVID-19 with a reduction of ICU admission and mortality. Further studies are urgently needed to establish the role of statins in patients with COVID-19. Such studies should assess the importance of pre-existing medical conditions as well as medications on clinical outcomes. These findings also highlight the need for systematic clinical studies to assess both pre- and in-hospital use of statins as the best means of reducing COVID-19 disease severity and mortality. Successful randomised clinical studies proving that statins offer a viable therapeutic option for COVID-19 disease might pave the way for their rapid deployment in the field as needed, due to their low cost, high availability, and well-established safety and tolerability profiles. Furthermore, the knowledge that we have gained over the past several months regarding COVID-19 and repurposing existing therapeutics such as statins will provide important insights and strategic measures to successfully control future epidemics and pandemics.
Conflict of interest
Maciej Banach: speakers bureau: Amgen, DaichiiSankyo, Esperion, Herbapol, KRKA, MSD, Mylan, Novartis, Novo-Nordisk, Sanofi-Aventis, Servier; consultant to Abbott Vascular, Akcea, Amgen, Daichii Sankyo, Esperion, Lilly, MSD, Resverlogix, Sanofi-Aventis; Grants from Amgen, Mylan, Sanofi and Valeant. All other authors have no conflict of interest.