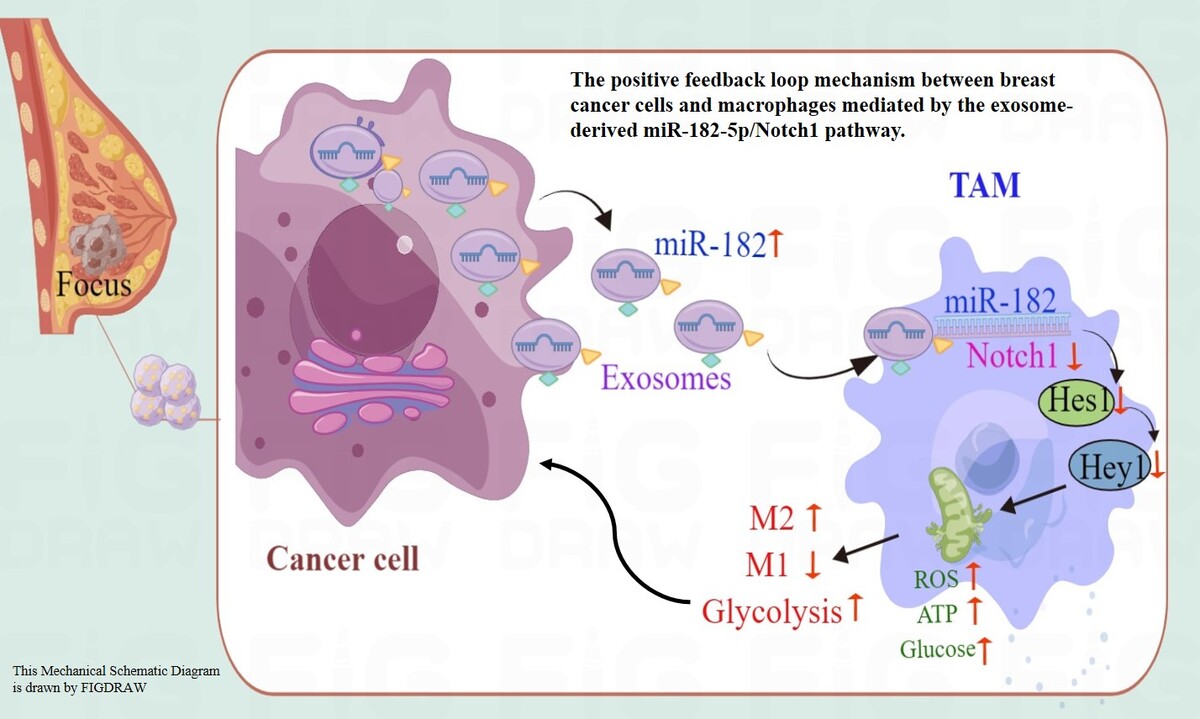
Introduction
The tumor microenvironment (TME) consists of non-cancerous cells and components within a tumor, including stromal cells and immune cells. The critical roles of the TME in tumor progression and responses to therapy, particularly immunotherapy, are well established [1, 2]. Notably, the infiltration of CD4+ T cells is recognized as a key factor in the efficacy of immunotherapy [3]. Recently, tumor-associated macrophages (TAMs), a significant component of the TME, have gained attention for their role in tumor progression and their potential as targets for clinical intervention [4]. Macrophages are categorized into M1 (classical) and M2 (alternative) phenotypes. M1 macrophages are associated with tumor-cytotoxic effects and antitumor immune responses, whereas M2 macrophages contribute to tissue repair and immunosuppression [5]. Dynamic shifts from M1 to M2 phenotypes in macrophages are observed in various inflammatory conditions and are linked to tumor-related tissue damage [6].
Numerous studies have demonstrated a correlation between the M2 polarization of tumor-associated macrophages (TAMs) and poorer prognosis in various human cancers [7]. Additionally, aberrant Notch pathway expression, identified in these malignancies, influences key cellular processes including proliferation, differentiation, apoptosis, and survival [8], as well as functions critical for immune regulation such as macrophage activation [9, 10].
MicroRNAs are small, 17-25-nucleotide, single-stranded, non-coding RNAs that primarily mediate post-transcriptional gene silencing by binding to the 3′-untranslated region (UTR) or the open reading frame (ORF) region of target mRNAs [11, 12]. The involvement of miRNAs in various biological activities, including cell proliferation, differentiation, and migration, as well as disease initiation and progression, particularly in tumor pathology, has been extensively documented [13]. For example, miR-325 targets lipocalin 15 to inhibit proliferation, migration and invasion of breast cancer cells [14]. Similarly, miR-610 functions as a tumor suppressor by reducing cisplatin resistance in hepatocellular carcinoma through the targeted silencing of hepatoma-derived growth factor [15]. Moreover, the down-regulation of miR-182 expression is associated with progression and metastasis of osteosarcoma [16]. Overexpression of microRNA-182 has been documented to be associated with metastasis and poor patient prognosis in breast cancer patients [13]. Functional studies have demonstrated that up-regulation of miR-182 in cancer cells significantly contributes to accelerated tumor proliferation, migration, invasion, epithelial-mesenchymal transition, metastasis, stemness, and therapy resistance, through the suppression of multiple genes [17–21]. In breast cancer, miR-182 has been shown to regulate trastuzumab resistance and tamoxifen sensitivity [19, 20]. Additionally, previous studies have demonstrated that breast cancer cells can induce miR-182 expression in macrophages, which in turn influences M2 polarization [15].
Exosomes, originating from the membranes of multivesicular bodies (MVB), have diameters ranging from 50 nm to 150 nm [22]. These vesicles are abundantly present in various body fluids and serve as vehicles for intracellular communication, transporting proteins, lipids, nucleic acids, and other substances [23]. Due to their ease of extraction and stability in biological environments, miRNA contents have emerged as preferred molecules in exosomes for research [22]. Recent studies have demonstrated the diagnostic potential of exosomal miRNA across a range of cancers, including bladder, ovarian, hepatoblastoma, colorectal, and glioblastoma [24, 25]. Specifically, the detection of certain mRNAs in exosomes aids not only in cancer diagnosis but also in evaluating the progression of the tumor microenvironment. Exosomes facilitate critical communication between tumor and non-tumor cells within the microenvironment, playing vital roles at different stages of tumor development, such as immune regulation, microenvironment remodeling, angiogenesis, invasion, and distant metastasis [26, 27]. For example, miR-126, delivered to the lungs by exosomes from MDA-MB-231 breast cancer cells, effectively inhibits the proliferation and migration of lung cancer cells, thereby suppressing tumor development [28].
While the contribution of miR-182-5p to the regulation of breast cancer cell malignancy and influence on M2 polarization of induced miR-182-5p expression in macrophages of breast cancer cells are well established, the precise mechanisms underlying how exosomal miR-182-5p from tumor cells influences macrophage polarization remain to be elucidated. This study aims to elucidate the mechanisms underlying the communication between cancer cells and macrophages during reprogramming, by employing exosomes from breast cancer cells with either overexpressed or inhibited miR-182-5p.
Material and methods
Clinical mRNA expression analysis
The miR-182-5p binding site on Notch1 was analyzed with the following parameters “has-miR-182-5p” and “Notch1” at the “miRNA-Target” section in the starBase database (starbase.sysu.edu.cn).
Clinical miR-182-5p and Notch1 mRNA expression in normal and breast cancer patients was also analyzed in the starBase database. Under the “Pan-Cancer” section, searching “has-miR-182-5p” in “miRNA Differential Expression”, or “Notch1” expression in “Gene Differential Expression”, with the parameter “Breast Invasive Carcinoma” in cancer type.
Clinical samples
This study used two treatment-naïve TNBC continuous cohorts: all treatment-naïve pathogenic confirmed female TNBC patients in Xinjiang Tumor Hospital from January 1, 2013 to October 1, 2015 and from January 1, 2022 to June 1, 2022 were included in the screening. We excluded patients without complete pathological or clinical information. Paraffin-embedded primary tumor specimens of 157 patients from January 1, 2013 to October 1, 2015 were used for immunohistochemical detection of Notch1, and frozen primary tumor and adjacent tissues of 30 patients from January 1, 2022 to June 1, 2022 were used for the analysis of biochemical markers and the expression of miR-182-5p. Samples were obtained with informed patient consent and approval by the Medical Ethics Committee of the Affiliated Tumor Hospital of Xinjiang Medical University (G-2019050).
Mouse experiments
The BALB/c nude mice were obtained from Hangzhou Medical College. For the fat-pad injection assays, 5 × 106 MDA-MB-231 cells were mixed with 100 µl of Matrigel and injected into the mammary fat pads on each side of 8-week-old female mice. After 7 days, miR-182-5p agomir, miR-182-5p antagomir, and the negative control oligonucleotides were injected into the tumors every 3 days for a total of 7 times. All animal studies were conducted according to the guidelines for the care and use of laboratory animals and were approved by the Medical Ethics Committee for Animal Experiments of the Affiliated Tumor Hospital of Xinjiang Medical University (IACUC-20190226-49).
Results
miR-182-5p and Notch1 expression in TNBC: analysis of database and clinical samples
Previous studies have already established the oncogenic roles of miR-182, which promotes cancer invasion by targeting the Notch1/Hes1 pathway and reprogramming M2 polarization of macrophages in cancer cells and mice. Utilizing the starBase database (starbase.sysu.edu.cn), the 3′UTR of Notch1 was found to have a binding site for miR-182-5p (Figure 1 A), indicating that has-miR-182-5p is predicted to interact directly with Notch1. To evaluate the relationship between miR-182-5p and Notch1 in breast cancer, the expression levels of miR-182-5p and Notch1 were analyzed using public databases and clinical breast cancer samples. A negative correlation in expression between miR-182-5p and Notch1 was demonstrated (Figures 1 B, C).
Figure 1
MiR-182-5p and Notch1 expression in clinical samples. A – 3′UTR of Notch1 had the binding site of miR-182-5p in starBase database (starbase.sysu.edu.cn). miR-182-5p showed overexpression (B) and Notch1 (C) showed low expression in breast cancer compared to normal tissue in starBase database. D – miR-182-5p was overexpressed in 30 TNBC samples. Numeric data are expressed as mean ± SEM. ***p < 0.001
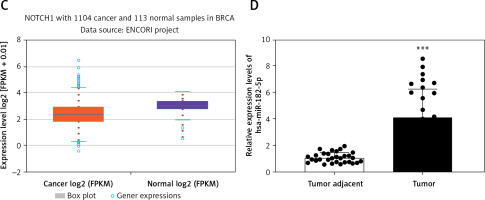
To further explore the impact of Notch1 expression, Notch1 was detected by IHC in 157 FFPE TNBC samples from Xinjiang Tumor Hospital. Notch1 expression was not associated with age, menstrual period, tumor size, or Ki67 expression but was significantly related to BMI, lymphatic metastasis, histological grade, and tumor stage (Table I). These results suggest that Notch1 expression is associated with metastasis but not with proliferation. Furthermore, overexpression of miR-182-5p was observed in 30 frozen TNBC samples compared to adjacent non-tumorous tissue (Figure 1 D). The biochemical indicators of glycolysis, including glucose (GLU), lactate (LD), lactate dehydrogenase (LDH), hexokinase (HK), pyruvate kinase (PK), and adenosine triphosphate (ATP), were notably elevated in TNBC (Supplementary Figure S1).
Table I
The relationship between Notch1 expression and clinical information of patients
Construction of exosomes from miR-182-5p overexpression/inhibition in MDA-MB-231 cells
To evaluate the regulatory effects of miR-182-5p in reprogramming macrophages, constructs for miR-182-5p mimics, miR-182-5p inhibitors, and corresponding controls were developed. Initially, miR-182-5p levels were quantified by qPCR in the human breast cancer cell line MDA-MB-231 and the normal human breast cell line MCF-10A. Our findings indicate that miR-182-5p was significantly overexpressed, and Notch1 expression was notably decreased in MDA-MB-231 cells (Figure 2 A). Following the transfection with miR-182-5p mimics and inhibitors, the expression of miR-182-5p altered in MDA-MB-231 cells in accordance with the plasmid (Figure 2 B) and in exosomes derived from transfected MDA-MB-231 cells (Figure 2 C).
Figure 2
Constructed exosomes from miR-182-5p overexpression/inhibition TNBC cell line. A – miR-182-5p showed overexpression and Notch1 showed low expression in MDA-MB-231 cell. The cell (B) and exosome (C) miR-182-5p expression changed significantly according to miR-182-5p mimics/inhibitor transfected to MDA-MB-231 cell. D – miR-182-5p was proved to combine with Notch1 directly by luciferase reporter assays. *miR- 182-5p mimics compared to mimics-NC. #miR-182- 5p inhibitor compare to inhibitor-NC. Numeric data are expressed as mean ± SEM. */#p < 0.05; **p < 0.01; ***p < 0.001
miR-182-5p regulates Notch1 by direct combination
To illustrate the mechanism of miR-182-5p regulating Notch1, wild-type and mutation Notch1 luciferase plasmid were constructed. By using luciferase, miR-182-5p mimics were proved to combine with wildtype-Notch1 directly, but not MT-Notch1 (Figure 2 D).
Regulative effects of exosomal miR-182-5p on macrophage reprogramming
M0 macrophages were co-cultured with exosomes from MDA-MB-231 cells transfected with either miR-182-5p mimics or inhibitors. The expression changes of key genes in the Notch1/Hes1 pathway, including Notch1, Hes1, HK2, and PKM2, were evaluated at both RNA and protein levels. Additionally, macrophage biomarkers and biochemical indicators of glycolysis were detected.
In summary, exosomes containing miR-182-5p mimics led to a reduction in the RNA (Figure 3 A) and protein (Figure 3 B) expression levels of Notch1 and Hes1. This was followed by upregulation of HK2 and PKM2 expression (Figures 3 A, B), which subsequently induced the polarization of macrophages towards the M2 phenotype (Figure 3 C) and an increase in glycolysis indicators, including GLU, LD, LDH, HK, PK, and ATP (Supplementary Figure S2). Conversely, exosomes with miR-182-5p inhibitors prompted upregulation in Notch1 and Hes1 expression, which in turn restrained HK2 and PKM2 expression (Figure 3 A, B). This induced polarization of macrophages towards the M1 phenotype (Figure 3 C), and led to a decrease in GLU, LD, LDH, HK, PK, and ATP (Supplementary Figure S2).
Figure 3
The effect of exosomes from miR-182-5p overexpression/inhibition TNBC cells on macrophage polarization. Notch1/Hes1 pathway RNA (A) and protein (B) expression in macrophages was significantly modified with TNBC exosomes containing miR-182-5p mimics/inhibitors. C – The M2 polarized macrophages transferred by TNBC exosome induction contain miR-182-5p mimics/inhibitors. *exo-miR-182-5p mimics compare to exo-mimics-NC. #exo-miR-182-5p inhibitor compare to exo-inhibitor-NC. Numeric data are expressed as mean ± SEM. */#p < 0.05; **/##p < 0.01; ***/###p < 0.001

Effects of exosomal miR-182-5p-induced macrophage regulation on cancer progression
Our findings demonstrate that exosomal miR-182-5p from breast cancer cells can induce M2 polarization of macrophages through regulation of the Notch1/Hes1 pathway, subsequently leading to variations in the biochemical indicators of glycolysis. However, the extent to which exosomal miR-182-5p-induced M2 polarization of macrophages and changes in the immune microenvironment affect breast cancer progression remains unclear.
To elucidate the impact of exosomal miR-182-5p-induced M2 polarization of macrophages on breast cancer progression, breast cancer cells (MDA-MB-231) were co-cultured with macrophages treated with exosomes. Co-culturing with macrophages treated with exo-miR-182-5p mimics significantly enhanced colony formation (Figure 4 A) and migration/invasion (Figure 4 B) and reduced apoptosis (Figure 4 C) of MDA-MB-231 cells. Conversely, co-culturing with macrophages treated with exo-miR-182-5p inhibitors significantly reduced colony formation (Figure 4 A) and migration/invasion (Figure 4 B) and increased apoptosis (Figure 4 C) of MDA-MB-231 cells.
Figure 4
The influence of exosomal miR-182-5p induced macrophage M2 polarization on breast cancer progression. Survival (A), migration/invasion (B), and apoptosis (C) of MDA-MB-231 were altered notably when co-cultured with exosomal miR-182-5p-induced macrophages. *M0-exo-miR-182-5p mimics compared to M0-exo-mimics-NC. #M0-exo-miR-182-5p inhibitor compared to M0-exo-inhibitor-NC. Numeric data are expressed as mean ± SEM. ***/###p < 0.001 The influence of exosomal miR-182-5p induced macrophage M2 polarization on breast cancer progression. Apoptosis (C) of MDA-MB-231 were altered notably when co-cultured with exosomal miR-182-5p-induced macrophages. *M0-exo-miR-182-5p mimics compared to M0-exo-mimics-NC. #M0-exo-miR-182-5p inhibitor compared to M0-exo-inhibitor-NC. Numeric data are expressed as mean ± SEM. ***/###p < 0.001

Effects of miR-182-5p regulation on breast cancer progression in vivo
To investigate the impact of miR-182-5p on breast cancer progression in vivo, a cell-derived xenograft (CDX) model using BALB/c nude mice was established. MDA-MB-231 cells were transfected with miR-182-5p-agomir (to overexpress miR-182-5p), miR-182-5p-antagomir (to inhibit miR-182-5p), and corresponding controls. Twenty-four hours after transfection, 5 × 106 cells were injected into the armpit of BALB/c nude mice. The diameter of tumors was measured every 5 days until day 44, at which point the mice were euthanized for further analysis.
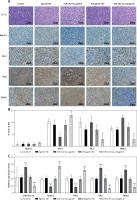
Tumor volume (Figure 5 A) and weight (Figure 5 B) significantly increased in the miR-182-5p-agomir treated mice, with proliferation confirmed by H&E staining (Figure 6 A). M2 macrophage polarization was notably upregulated (Figure 5 C). Conversely, tumor volume (Figure 5 A), weight (Figure 5 B), and proliferation (Figure 6 A) significantly significant shrunk in the miR-182-5p-antagomir treated mice. M2 macrophage polarization was notably downregulated (Figure 5 C).
Figure 5
The phenotype shifted by miR-182-5p overexpression/inhibition in vivo. The tumor volume (A), tumor weight (B). #miR-182-5p-antagomir compared to antagomir-NC. Numeric data are expressed as mean ± SEM. */#p < 0.05; **p < 0.01; ***/###p < 0.001 The phenotype shifted by miR-182-5p overexpression/inhibition in vivo. Macrophage polarization (C) were shifted vitally in the miR-182-5p agomir/antagomir nude-mouse model. *miR-182-5p-agomir compared to agomir-NC. #miR-182-5p-antagomir compared to antagomir-NC. Numeric data are expressed as mean ± SEM. */#p < 0.05; **p < 0.01; ***/###p < 0.001
Furthermore, the protein (Figure 6 A, B) and RNA (Figure 6 C) levels of Notch1, Hes1, HK2, and PKM2 changed in a manner consistent with the in vitro findings, leading to changes in biochemical indicators (Supplementary Figure S3). There was one inconsistency: the protein levels of Notch1 did not show a clear trend, possibly due to ineffective staining by the Notch1 IHC antibody.
Figure 6
The mechanism switched by miR-182-5p overexpression/inhibition in vivo. The proliferation by H&E (A), as well as protein (B) and RNA (C) of Notch/Hes1 pathway were re-programmed outstandingly by miR-182-5p agomir/antagomir in nude-mouse model. *miR-182-5p-agomir compared to agomir-NC. #miR-182-5p-antagomir compared to antagomir-NC. Numeric data are expressed as mean ± SEM. */#p < 0.05; ##p < 0.01; ***p < 0.001
Discussion
Notch family proteins, serving as transmembrane receptors, play a pivotal role in determining cell fate by regulating differentiation, apoptosis, and proliferation. Aberrant expression of the Notch pathway, observed in various human cancers, influences proliferation, differentiation, apoptosis, and survival [8]. Specifically, elevated Notch expression has been correlated with poorer breast cancer outcomes [29]. The Notch family consists of four highly conserved transmembrane receptors, Notch1, Notch2, Notch3, and Notch4, exhibiting variable expression levels across different breast cancer genotypes [30]. Notch2 overexpression promotes tumor progression in estrogen receptor-positive luminal tumors [31], while Notch3 is implicated in regulating proliferation in HER2-negative breast cancer [32]. Additionally, Notch4 activation disrupts ductal and lobular growth, thereby inducing tumorigenesis [29]. Most studies focus on the Notch1 signaling pathway, which has been shown to govern migration and metastasis, and its inhibition reduces these processes [33]. A meta-analysis involving 4,463 patients across 17 studies found a negative correlation between increased Notch1 levels and recurrence-free survival, regardless of other prognostic factors or cancer type [34]. Notch1’s critical role extends to resistance mechanisms against paclitaxel, suggesting its potential as a therapeutic target [35]. High Notch1 expression levels have been linked to reduced overall survival in breast cancer patients [36], particularly in HER2-positive breast cancer, where Notch1 enhances stem cell survival, contributing to trastuzumab resistance [37]. Notch1’s role in triple-negative breast cancer (TNBC) is particularly notable, with higher mRNA expression levels significantly associated with TNBC, but not with ER-negative samples [38]. Notch1 activation of the AKT pathway and promotion of EMT through MVP activation underscore its role in chemo-resistance in TNBC [39].
Several studies have reported regulatory mechanisms between miRNA and Notch1 in cancer cells. For instance, hsa-miR-599 downregulation in breast cancer deactivates the BRD4/Jagged1/Notch1 axis, thus inhibiting cancer progression [40]. MiR-34a suppresses breast cancer cell proliferation and invasion, potentially through targeting Notch1 [41]. Overexpression of OIP5 inhibits hsa-miR-139-5p, leading to the de-repression of NOTCH1, a core oncogene in breast cancer progression [42]. EVs carrying miR-887-3p target BTBD7 and activate the Notch1/Hes1 signaling pathway, promoting drug resistance in breast cancer cells [43]. Previous research has established the importance of miRNAs in breast cancer through the Notch1 pathway. However, few studies have explored miRNA/Notch1 regulation of macrophage polarization. In breast cancer, one study investigated miRNA affecting macrophage polarization via the microRNA-146a-5p/NOTCH1 axis [44]. Nonetheless, the precise role and mechanism of miRNA in regulating Notch1 to reprogram M2 macrophage polarization remain unclear.
This study demonstrated that exosomal miR-182-5p reprograms macrophage polarization from M1 to M2 by reducing Notch1 expression, thereby enhancing breast cancer progression both in vitro and in vivo. Through bioinformatics analysis, we identified a direct interaction between miRNA-182-5p and the Notch1 3′UTR (Figure 1 A), confirmed by a luciferase assay (Figure 2 D). Clinical analysis showed a correlation between miR-182-5p expression and Notch1 expression in human tumor tissues (Figures 1 B–D). After transfection with miR-182-5p mimics, inhibitors, and controls for 24 h (Figure 2 B), exosomes were extracted from transfected breast cancer cells (MDA-MB-231) and quantified for miR-182-5p expression by RT-qPCR (Figure 2 C). Macrophages co-cultured with these exosomes exhibited altered Notch1/Hes1 pathway expression (Figure 3 A, B), resulting in changes in polarization (Figure 3 C), and modifications of biochemical indicators (Supplementary Figure S2). Furthermore, breast cancer cells co-cultured with reprogrammed M2 macrophages showed increased colony formation (Figure 4 A), migration, and invasion (Figure 4 B), as well as reduced apoptosis (Figure 4 C). These findings were validated in a BALB/c nude-mouse model in vivo (Figures 5, 6). This study is the first to elucidate the breast cancer-macrophage feedback loop mechanism via the exosome-miR-182-5p/Notch1 pathway for macrophage reprogramming. Based on our findings, exosomal miR-182-5p inhibitors could be used as anti-tumor drugs for TNBC by regulating macrophage polarization.
Exosomal miRNAs have been found to play a positive role in antitumor immunity. Exosomes derived from breast cancer deliver miR-130 and miR-33, which downregulate TGF-β and IL-10, ultimately inducing the polarization of M1 macrophages [45]. Human HPV+ NHSCC-derived exosomes have been shown to transport miR-9 to macrophages, inducing M1 macrophage polarization and increasing tumor radiosensitivity [46]. Moreover, miR-1827 delivered by exosomes from human umbilical cord mesenchymal stem cells (MSCs) has been shown to inhibit macrophage M2 polarization and prevent colorectal liver metastasis [47]. Furthermore, exosomes represent an ideal delivery system, offering many advantages including low immunogenicity, high stability, and targeted delivery capability [26]. Numerus studies have demonstrated that exosomes hold promise as a reliable vector for gene therapy in cancer treatment. For instance, exosomes loaded with glucose-regulated protein 78 siRNA have been shown to overcome tumor cell resistance to sorafenib in hepatocellular carcinoma [48]. Similarly, exosome-mediated siRNA delivery has been effective in reducing S100A4 expression, significantly inhibiting postoperative metastasis in triple-negative breast cancer [49]. In summary, employing an exosomal miR-182 inhibitor could represent a viable therapeutic strategy for TNBC.
Even though using exosomal miR-182-5p inhibitors as anti-tumor drugs is still far from clinical application, drugs that regulate macrophage polarization may hold promise for breast cancer treatment. CSF1R signaling is vital for the differentiation and survival of the mononuclear phagocyte system, particularly macrophages [50], making CSF1R signaling in TAMs a compelling therapeutic target. Regorafenib, an antiangiogenesis inhibitor, has been shown to reduce tumor-associated macrophages and potently inhibit CSF1R, with proven efficacy in advanced colorectal cancer (CRC), gastrointestinal stromal tumors (GIST), and hepatocellular carcinoma (HCC) [51]. Several small molecules such as pimicotinib (ABSK021), ARRY-382, PLX7486, BLZ945, and JNJ40346527 and monoclonal antibodies (mAbs) including emactuzumab, AMG820, IMC-CS4, cabiralizumab, MCS110, and PD-0360324, targeting CSF1R or CSF1, have emerged as a promising anti-tumor strategy.
In conclusion, Overall, our study highlights the role of exosomal miR-182-5p from breast cancer cells in reprogramming M2 macrophage polarization by targeting Notch1, leading to accelerated tumor progression through regenerative feedback. Our findings suggest that inhibiting miR-182-5p in TAMs offers a potential strategy for targeting these cells, emphasizing the effectiveness of miR-182-5p-inhibiting RNA interference (RNAi) therapy. Although RNAi therapy remains a long-term goal in cancer treatment, drugs targeting macrophage polarization, such as CSF1R inhibitors, may offer alternative and immediate therapeutic options for breast cancer patients with miR-182-5p overexpression. Further prospective and interventional clinical studies are necessary to validate miR-182-5p’s potential as both a prognostic marker and a therapeutic target in cancer.