Infertility remains a global health concern, affecting over 50 million couples worldwide [1]. Among men, azoospermia – a condition characterized by the complete absence of sperm in the ejaculate – is observed in approximately 1% of the general male population and accounts for about 10–15% of all male infertility cases [1, 2]. It is diagnosed following two independent semen analyses that confirm the absence of spermatozoa [3]. Non-obstructive azoospermia (NOA), unlike its obstructive counterpart, results from impaired sperm production due to hypothalamic-pituitary-testicular dysfunction [1, 3, 4].
The clinical presentation of NOA often includes small, soft testes, elevated gonadotropin levels – particularly follicle-stimulating hormone (FSH) – and diminished serum testosterone. Yet behind this consistent clinical picture lies a surprisingly diverse set of underlying causes. Some men may have genetic abnormalities such as Klinefelter syndrome or Y-chromosome microdeletions, while others present with idiopathic forms where no clear etiology is found. Congenital factors (e.g. cryptorchidism) and post-infectious complications (e.g. mumps orchitis) are also common contributors. And then there are environmental culprits – chemotherapy, radiotherapy, or even a past vasectomy – that quietly influence testicular function over time [1–4].
Interestingly, microsurgical testicular sperm extraction (micro-TESE) has emerged not just as a treatment, but almost as a diagnostic strategy in itself. It is now considered standard practice in men with NOA, with reported sperm retrieval rates approaching 50% [1, 5]. However, success does not happen in a vacuum. A solid preoperative evaluation is essential – often involving genetic workups for karyotype and Y-microdeletions, ultrasound to check for anatomical variations, and a detailed hormonal panel [5, 6].
Before surgery, some clinicians explore hormonal therapies as a way to improve outcomes, although the consensus on efficacy varies. Common options include clomiphene citrate, human chorionic gonadotropin (hCG), anastrozole, and even exogenous FSH itself [2]. Still, these treatments remain adjunctive and are often selected case by case.
Among the many variables that have been studied – testis size, inhibin B, and endocrine markers – histopathology continues to stand out as the most consistent predictor of sperm retrieval success [6–9]. Particularly when the initial micro-TESE fails, a careful review of the biopsy findings can shed light on whether a second attempt is worthwhile.
Looking closer at testicular tissue under the microscope, several distinct patterns emerge. Some men exhibit Sertoli cell-only syndrome (SCOS), where the seminiferous tubules lack all germ cells and contain only Sertoli cells – a grim prognosis in many cases [1, 5]. Others show hypospermatogenesis, which is a more favorable finding, with all stages of germ cell development present but in reduced numbers. This pattern appears not only in azoospermic individuals but also among those with severe oligozoospermia. Then there is germ cell maturation arrest (GCMA), where development is halted – often at the level of primary spermatocytes or late spermatids. Seminiferous tubule hyalinization and interstitial fibrosis, also observable in some biopsies, typically indicate long-standing or irreversible damage [5–9].
In rare cases, areas of preserved spermatogenesis are still present despite systemic markers indicating otherwise. These focal zones – missed in conventional sampling – are often the target in micro-TESE, making surgical precision and histological insight essential allies in male infertility care [1, 2]. In patients with hyalinized tubules, testicular tissue shows extensive intratubular and peritubular fibrosis with no germ cells present. This condition is often accompanied by high serum FSH and significantly reduced testicular volume [10–17].
This study explores the association between histopathological findings and outcomes of “second-look” microsurgical sperm retrieval attempts following an initial failed micro-TESE. Identifying favorable histological patterns may improve clinical decision-making and patient counseling.
Methods
Patient population
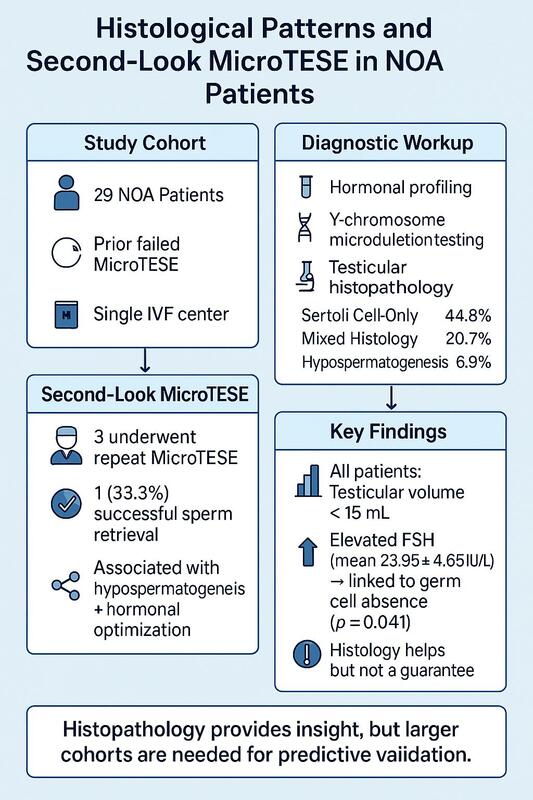
This study involved a cohort of 29 men diagnosed with NOA, all of whom had previously undergone unsuccessful microsurgical testicular sperm extraction procedures at a single in-vitro fertilization center. These patients constituted a specific subgroup within the broader population of 114 individuals who underwent micro-TESE at the center between [Jan 2020–Dec 2024]. The overall sperm retrieval success rate during this period was 74.6% (85 out of 114 cases), while 25.4% (29 out of 114) represented failed retrievals and formed the basis of this retrospective analysis. Conventional TESE procedures were not routinely performed at the facility during the study period; all patients were managed using standardized micro-TESE techniques.
Azoospermia was confirmed through analysis of two independently collected and centrifuged semen samples, both of which showed a complete absence of spermatozoa. A total of 14 patients were excluded from the initial micro-TESE cohort. This included 7 men with confirmed obstructive azoospermia, based on clinical and ultrasound criteria, and 7 patients with complete microdeletions in the AZFa (n = 2) or AZFb (n = 5) regions of the Y chromosome. These cases were excluded prior to the retrospective analysis in accordance with standard clinical guidelines.
Inclusion criteria for second-look micro-TESE.
Second-look micro-TESE was offered selectively to patients whose initial histopathological evaluation demonstrated the presence of germ cells, specifically those diagnosed with hypospermatogenesis or mixed histological patterns that included areas of preserved spermatogenesis. These patterns were identified as having potential for residual sperm production. Patients with histological findings consistent with complete SCOS, hyalinized seminiferous tubules, or complete maturation arrest without evidence of spermatogenic activity were excluded from repeat procedures, in accordance with clinical best practices. The decision for second-look intervention also considered the patient’s hormonal profile, recovery period, and overall reproductive plan.
Patient evaluation
To establish the diagnosis of NOA, each patient underwent a comprehensive evaluation that included medical history, physical examination, semen analysis, hormonal profiling, and genetic testing. Historical data collected encompassed age, smoking status, height, weight, and records of any previous medical or surgical interventions. Testicular volume was assessed through a combination of physical examination and scrotal color Doppler ultrasound, which also aided in detecting anatomical anomalies [1].
Laboratory and genetic assessment
The hormonal profile included measurements of serum luteinizing hormone (LH), testosterone (T), and follicle-stimulating hormone (FSH). Genetic analysis involved standard karyotyping alongside targeted screening for Y-chromosome microdeletions, focusing specifically on the AZFa, AZFb, and AZFc regions [1, 5].
Surgical procedure
The microsurgical testicular sperm extraction procedure was performed under general anesthesia following the technique originally described by Schlegel [18]. In the absence of a significant volume discrepancy between the testes, the right testis or the one with greater volume was selected for the initial approach. A single longitudinal incision was made along the scrotal median raphe to deliver the testis. Once exposed, the tunica vaginalis was carefully opened to access the tunica albuginea.
Using an operating microscope at 20× and 40× magnification, the testicular parenchyma was meticulously inspected for dilated or opaque seminiferous tubules, which were presumed to have higher potential for active spermatogenesis. These selected tubules were carefully excised and submitted for sperm retrieval attempts. If no viable spermatozoa were found in the initial testis, the contralateral testis was explored using the same technique to increase the likelihood of successful retrieval [5].
The procedure was concluded when either spermatozoa were successfully retrieved or further dissection was deemed unproductive. A representative sample of the testicular tissue was preserved in Bouin’s solution for subsequent histopathological analysis. Hemostasis was achieved, and the incision in the tunica albuginea was closed using an absorbable 5-0 suture. The scrotal layers were then anatomically re-approximated and sutured in layers to ensure proper wound healing [5].
Histopathological assessment
Histological examination of the testicular biopsy specimens was conducted by a single experienced pathologist to ensure consistency in evaluation. Bilateral testicular biopsies were obtained following the micro-TESE procedure, with tissue samples prepared for analysis using standard hematoxylin and eosin (H&E) staining. The stained sections were examined under a light microscope to assess various structural and cellular parameters critical to spermatogenic activity [1].
Key histological features evaluated included the density and arrangement of germ cells, the presence and architecture of seminiferous tubules (with attention to both their size and quantity), the thickness of the basement membrane, and the distribution of Sertoli and Leydig cells. Particular attention was also paid to signs of tubular hyalinization and other degenerative changes. These features were used to classify the histological patterns observed in the testicular tissue.
Several distinct histopathological categories were identified: hypospermatogenesis, SCOS, seminiferous tubule hyalinization, and GCMA. In cases where more than one pathological pattern was present within the same biopsy specimen, the findings were categorized as “mixed patterns.” These mixed profiles typically represented a combination of degenerative and partially preserved spermatogenic activity within different regions of the testicular tissue [5].
Statistical analysis
Statistical analyses were conducted using IBM SPSS version 23.0 (IBM Corp., Armonk, NY). Descriptive statistics were employed to summarize and present the data, including means and standard deviations for continuous variables. To compare continuous variables between groups, Student’s t-test was applied. A p-value of less than 0.05 was considered statistically significant.
Histological images were not included due to ethical and institutional limitations on retrospective specimen use without explicit visual data consent. All diagnoses were performed by a senior uropathologist following standardized histological classification criteria.
Results
The mean age was 34.6 ±5.2 years. Most participants were married (79.3%), while 20.7% were unmarried. Educational attainment varied, with the majority holding a bachelor’s degree (58.6%), followed by high school education (31.0%) and master’s degrees (10.4%). In terms of income distribution, 48.3% of participants were classified within the middle-income group, 37.9% fell into the low-income category, and 13.8% were part of the high-income group. Employment status further reflected diverse socioeconomic backgrounds, with 69.0% employed, 20.7% self-employed, and 10.4% unemployed.
Most individuals were non-smokers (72.4%) and reported a sedentary lifestyle (62.1%). Over half of the cohort (51.7%) were classified as overweight based on their body mass index. Among reported medical conditions, hypertension emerged as the most prevalent, affecting 13.8% of participants. Additionally, 58.6% had received medical therapy as their primary form of previous treatment for infertility.
Table I summarizes the histopathological findings among the study participants. SCOS was the most frequently observed pattern, present in 13 (44.8%) individuals. A mixed histological pattern, indicating the coexistence of multiple abnormalities within the same biopsy, was identified in 6 (20.7%) participants. GCMA and seminiferous tubule hyalinization were each found in 4 (13.8%) participants. Hypospermatogenesis was the least common finding, detected in only 2 (6.9%) participants.
Table I
Histopathological findings in study participants
The mixed pattern category encompassed specific combinations of histopathological abnormalities. Among the 6 participants identified with mixed patterns, one individual (16.7%) demonstrated a combination of SCOS and hypospermatogenesis. Three participants (50.0%) exhibited both SCOS and GCMA, while the remaining 2 (33.3%) showed a combination of seminiferous tubule hyalinization and GCMA.
Among the 29 patients included in the final analysis, 5 (17.2%) were found to have Y-chromosome microdeletions, all within the AZFc region. No deletions were detected in the AZFa or AZFb regions among this cohort, in line with the exclusion criteria. One of the 3 patients who underwent a second-look micro-TESE had an AZFc deletion, and was included based on histological evidence of hypospermatogenesis.
Table II outlines the clinical and hormonal profiles of participants who underwent a second-look micro-TESE procedure. Out of the total study sample, only 3 (10.3%) patients proceeded with a second-look attempt. Of these, 2 (66.7%) individuals exhibited hypospermatogenesis, while 1 (33.3%) patient showed a mixed histological pattern involving both hypospermatogenesis and SCOS. Sperm retrieval was successful in 1 (33.3%) case, indicating a positive outcome. Postoperative histopathological examination revealed SCOS in two of the 3 second-look patients (66.7%).
Table II
Clinical and hormonal profiles following second-look micro-TESE procedure among study participants
Hormonal assessments revealed a notable difference in follicle-stimulating hormone (FSH) levels between participants with and without detectable germ cells. Patients exhibiting germ cells had a mean FSH level of 17.85 ±3.50 IU/l, while those without evidence of spermatogenesis demonstrated a significantly higher mean FSH level of 23.95 ±4.65 IU/l (p = 0.041). In contrast, serum testosterone levels were relatively similar between the two groups, averaging 14.90 ±1.25 nmol/l in patients with germ cells and 13.75 ±2.85 nmol/l in those without, a difference that did not reach statistical significance (p = 0.522).
Scrotal ultrasound evaluations indicated uniformly reduced testicular volume among participants, with all measurements falling below 15 ml. The mean volume of the right testis was 10.50 ml, while the left averaged 9.20 ml. Genetic analysis revealed that all patients possessed a normal 46,XY karyotype.
Discussion
The journey toward understanding male infertility often begins with a definition – infertility is generally recognized as the inability to conceive after 1 year of regular, unprotected intercourse. But definitions alone fail to capture the complexity of diagnoses such as NOA, which continues to challenge clinicians due to its wide-ranging etiologies and unpredictable treatment outcomes. In fact, much of the difficulty lies not in labeling the condition, but in interpreting what underlies it. Most cases of NOA trace back to either disruptions in endocrine signaling or intrinsic testicular failure – two distinct but converging pathways [1].
Clinical markers do not always shout–they often whisper. Subtle signs such as smaller testicles, slightly elevated FSH, or a silent microdeletion in the Y-chromosome may already signal NOA before a diagnosis is confirmed [1]. And while such indicators may seem routine, they are diagnostically powerful. One model suggests that combining a testicular long axis of less than 4.6 cm with an FSH above 7.6 mIU/ml can predict NOA in nearly 9 out of 10 cases [19]. Conversely, a different pattern – a larger testis and lower FSH – usually tilts suspicion toward obstructive causes. These contrasts help shape the current practice of skipping invasive diagnostic biopsies in favor of proceeding directly to microsurgical sperm retrieval and cryopreservation for assisted reproductive technologies such as intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF) [3, 4, 19].
When Schlegel introduced micro-TESE in 1999, it was not just an upgrade from traditional TESE – it was a shift in thinking [18]. The old method relied on random sampling, which often missed the few islands of spermatogenesis hiding in the testicular landscape. Micro-TESE changed that by using visual cues under high magnification to pinpoint dilated seminiferous tubules more likely to be productive. And it worked. Retrieval rates jumped from the modest range of 16.7–45% seen with conventional TESE to a more promising 42.9–63% when guided by tubule morphology [20].
Nevertheless, the challenge of reliably predicting outcomes remains. While complete AZFa or AZFb deletions on the Y chromosome remain definitive negative predictors of sperm retrieval [20], there are no universally accepted positive markers. Though testicular volume and FSH have traditionally been explored as prognostic factors, our study – like others – found that elevated FSH and reduced testicular volume did not necessarily predict negative outcomes. For instance, sperm retrieval was successful in one of three second-look cases, despite uniformly low testicular volume and elevated FSH, suggesting that these parameters alone should not exclude patients from a second attempt [1].
Testicular histopathology, on the other hand, continues to emerge as a more consistent indicator. Since biopsies are now typically taken during micro-TESE procedures and not beforehand, the initial histological findings can become the most practical guide for determining the viability of a second-look operation [1]. Among our patients, hypospermatogenesis – defined by the presence of all germ cell stages, albeit in reduced numbers – was noted in both isolated and mixed patterns. While relatively uncommon in our cohort (6.9%), its presence in two of the three second-look patients, including the only successful sperm retrieval, highlights its predictive relevance. This pattern has been linked to androgen resistance, congenital germ cell deficits, and exposure to gonadotoxic factors. Its reported incidence varies widely, from 13% to over 50%, depending on the clinical setting and classification thresholds [13].
The most frequent histological diagnosis in our study was SCOS, observed in 44.8% of participants. SCOS is histologically defined by seminiferous tubules devoid of germ cells, containing only Sertoli and Leydig cells. It is generally considered irreversible and is often associated with conditions such as cryptorchidism, chronic liver disease, prior radiation or chemotherapy, and hormonal imbalances [15].
GCMA, characterized by the developmental interruption of germ cells at either pre-meiotic or post-meiotic stages, was seen in 13.8% of our cohort. Our findings fall within the 12–28% incidence range previously reported [13]. GCMA is often associated with genetic mutations, hypogonadotropic hypogonadism, or environmental and substance exposures [15].
Seminiferous tubule hyalinization was also present in 13.8% of patients. This form, often regarded as an “end-stage” pattern, is characterized by extensive peritubular and intratubular fibrosis and absence of spermatogenesis. It is frequently observed in patients with Klinefelter syndrome and other chromosomal abnormalities [13]. Although our findings align with previous literature, some studies have reported lower frequencies [17].
One of the key strengths of this study lies in its methodological consistency, with all biopsies analyzed by the same experienced uropathologist. While the relatively small sample size limits generalizability, this research offers valuable regional insight into the histopathological spectrum of NOA and informs decisions regarding second-look procedures after initial failure.
This study is limited primarily by its small sample size, particularly the very low number of patients (n = 3) who underwent a second-look micro-TESE. This restricts the statistical power and generalizability of our findings. Additionally, the retrospective nature of the study and single-center design may introduce selection bias and limit the broader applicability of the results. No long-term follow-up data on fertility outcomes or live births were collected, which further constrains the clinical impact. Future studies should incorporate larger, prospective cohorts and include outcome measures beyond sperm retrieval to assess the true clinical utility of second-look procedures. Histological images were not included due to ethical and institutional limitations on retrospective specimen use without explicit visual data consent.
In conclusion, this study highlights the importance of testicular histopathology in guiding decisions after failed micro-TESE in men with NOA. Patients with hypospermatogenesis or mixed patterns containing germ cells may still have a chance of successful sperm retrieval upon second-look procedures. However, our findings clearly demonstrate that even the presence of such histological patterns does not guarantee a positive outcome, as only one of three re-attempts was successful. These findings remain preliminary and exploratory in nature. We emphasize that eligibility for second-look procedures should be based on individualized histological and hormonal evaluation. The limited success rate of 1 out of 3 cases undergoing second-look micro-TESE underscores that even favorable histological patterns such as hypospermatogenesis do not ensure positive outcomes. Larger, multicenter studies are required to confirm these early insights and improve guidance for clinical practice.