Breast cancer molecular subtypes guide precision oncology [1], yet treatment gaps remain. While hormone receptor-positive (HR+) cases respond to endocrine therapy and human epidermal growth factor receptor 2-positive (HER2+) tumors respond to agents like trastuzumab [2], approximately 15–20% of cases classified as triple-negative breast cancer (TNBC) face limited therapeutic options, and these patients remain predominantly dependent on conventional chemotherapy.
Circular RNA (circRNA), a novel class of non-coding RNA molecules, serve as critical regulators of gene expression through diverse mechanisms. CircRNA orchestrate the initiation and progression of breast cancer by functioning as oncogenes or tumor suppressors. Their aberrant expression is implicated in multiple cancer hallmarks, such as dysregulated proliferation, apoptosis, autophagy, metastasis, and treatment resistance [3, 4].
Prior findings showed elevated circINTS4 in TNBC vs normal cells [5], suggesting prognostic/chemoresistance potential. Unvalidated clinically, this study bridges this gap by: (1) profiling subtype-specific circINTS4 expression patterns across tumor tissues; (2) correlating levels with progression-free survival (PFS) and overall survival (OS) outcomes to validate biomarker utility.
Methods
Study subjects
This study used surgical specimens from breast cancer patients treated at Liuyang People’s Hospital (2023). The tissue specimens were collected as follows: tumor tissues were obtained from surgical resections, normal breast tissues were collected from regions adjacent to benign breast lesions during surgical procedures, with adjacent normal tissues defined as histologically confirmed non-neoplastic breast parenchyma located > 5 cm from the tumor margin. All specimens were rinsed with saline, snap-frozen in liquid nitrogen, and stored at –80°C. The diagnosis of all tissue samples was confirmed by two independent pathologists who were blinded to the patients’ clinical data. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Hospital Ethics Committee (No. 2023-001). Written informed consent was obtained from all participants.
Inclusion and exclusion criteria.
Eligible participants were female patients aged 18–75 years with pathologically confirmed breast cancer, who had received no prior anticancer treatment (including neoadjuvant chemotherapy, endocrine therapy, targeted therapy, or radiotherapy) and were able to comply with regular follow-up examinations. Exclusion criteria consisted of receipt of neoadjuvant therapy, presence of distant metastasis at diagnosis, history of other malignancies, severe systemic comorbidities (e.g., advanced cardiovascular/cerebrovascular diseases, end-stage hepatic/renal dysfunction, severe chronic pulmonary conditions, or HIV infection), inability to complete the prescribed treatment regimen or unplanned therapy discontinuation, and male breast cancer patients.
Grouping criteria
We established seven molecular subtype cohorts: TNBC, n = 10, HR-positive/HER2-negative (HR+/HER2-, n = 10), HR-positive/HER2-positive (HR+/HER2+, n = 10), HR-negative/HER2-positive (HR-/HER2+, n = 10), ductal carcinoma in situ (DCIS, n = 10), TNBC-adjacent normal tissue (TNBC-NAT, n = 10), and normal breast tissue (B-NML, n = 10). Supplementary groups included: HER2 status (HER2+ vs HER2-; n = 20 each), TNBC vs TNBC-NAT (n = 10 each), and clinicopathological analyses (tumor size ≤ 2 cm [n = 16] vs. > 2 cm [n = 16], lymph node metastasis [n = 20] vs. none [n = 20], histological grade II [n = 10] vs. III [n = 10], lymphovascular invasion (LVI) (negative [n = 17] vs. positive [n = 17]). No significant differences in key anthropometrics (e.g., age, height, and weight) were observed among the compared patient groups. All breast cancer patients in these groups had ductal carcinoma in situ (DCIS) or AJCC Stage I/II invasive carcinoma; no advanced-stage cases were included. In our study, HER2-positive (HER2+) and HER2-negative (HER2-) groups were defined strictly according to the ASCO/CAP guidelines: HER2+ was defined as IHC 3+ or IHC 2+ with a positive ISH result, while HER2- was defined as IHC 0, IHC 1+, or IHC 2+ with a negative ISH result. Samples were consecutively collected without artificial matching; no baseline differences existed; all participants underwent PFS and OS monitoring. (PFS was defined as the time from the date of surgery to the first occurrence of either radiological disease progression or death from any cause, while OS was defined as the time from surgery to death from any cause.)
Experimental method
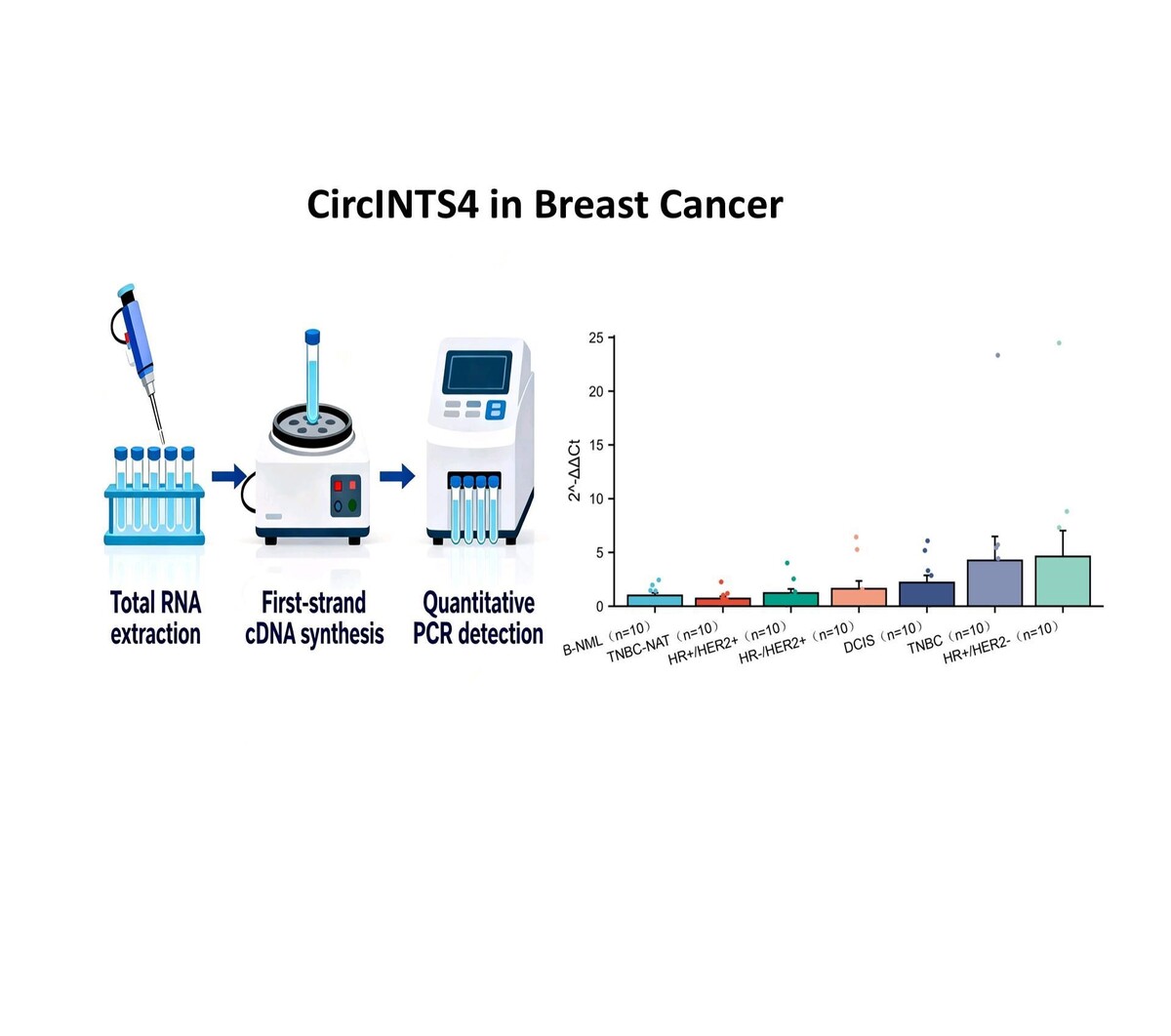
The experimental procedures will be performed in the following sequence: total RNA extraction, first-strand cDNA synthesis, and quantitative PCR detection. Primer sequences were designed as follows: circINTS4: Forward 5’-GAAGATGAGATGTATGGGCTC-3’, Reverse 5’-AAGTTCCTTGGCACGCTCAT-3’. β-actin (internal control): Forward 5’-AGGGCCGGACTCGTCATACT-3’, Reverse 5’-GGCGGCACCACCATGTACCCT-3’. The relative expression of the target genes was calculated using the comparative 2–ΔΔCt method, where Ct values were normalized to the endogenous control gene, and the fold change was determined by comparing the ΔCt values of the experimental group to those of the control group. No statistically significant differences were observed in key anthropometric data (including age, height, and weight) among the compared patient groups.
Statistical analysis
Statistical analyses were performed using SPSS (v26.0). Continuous data are expressed as mean ± standard deviation or median (interquartile range), and categorical variables as counts (percentages). The Shapiro-Wilk test was used to assess normality. For two-group comparisons, an unpaired t-test (normal distribution) or Mann-Whitney U test (non-normal distribution) was applied. For multi-group comparisons, one-way ANOVA or Kruskal-Wallis test with Dunn’s post hoc correction was used. A two-sided p-value < 0.05 was considered significant.
Results
CircINTS4 expression was analyzed via qRT-PCR in 70 breast tissues (B-NML, TNBC-NAT, HR+/HER2+, HR-/HER2+, DCIS, TNBC, HR+/HER2-), normalized to B-NML. Highest expression occurred in HR+/HER2- (4.62-fold) and TNBC (4.26-fold). However, no significant differences were found compared to normal tissue (all p > 0.05; Figure 1 A).
Figure 1
Expression profiling of circINTS4 in breast cancer subtypes. A – Differential expression of circINTS4 in human breast tissue specimens. B – Expression of circINTS4 in HER2-positive and HER2-negative breast cancer tissues. C – Expression of circINTS4 in TNBC and TNBC-adjacent normal tissue. *p < 0.05; ns, not significant. The relative expression of circINTS4 was calculated using the 2–ΔΔCt method
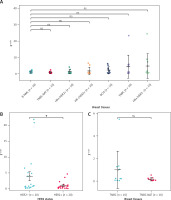
Stratified by HER2 status (HER2- vs HER2+, n = 20 each), circINTS4 expression was significantly elevated in HER2- tumors (3.97-fold vs 1-fold in HER2+ controls; p < 0.05) (Figure 1 B).
circINTS4 expression was lower in TNBC-adjacent normal tissues (TNBC-NAT, n = 10) than in TNBC samples (n = 10) (fold change: 0.17 vs. 1.0; p = 0.13), though this difference was not statistically significant (Figure 1 C).
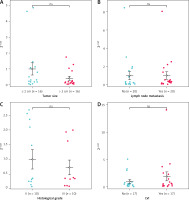
Stratifying patients by tumor size (≤ 2 cm [n = 16] vs. > 2 cm [n = 16]), lymph node status (negative [n = 20] vs. positive [n = 20]), histological grade (II [n = 10] vs. III [n = 10]), and LVI (negative [n = 17] vs. positive [n = 17]), all groups showed comparable baseline clinicopathological characteristics. No significant differences in circINTS4 expression were detected between comparison groups (Figures 2 A–D).
Figure 2
The relationship between circINTS4 and clinicopathological characteristics includes tumor size (A), lymph node metastasis (B), histological grade (C), and lymphovascular invasion, LVI (D). The relative expression of circINTS4 was calculated using the 2–ΔΔCt method
All patients were followed postoperatively (median: 22 months, range: 15–27). No local recurrence or distant metastasis occurred, as confirmed by quarterly imaging (ultrasound/MRI + systemic CT) and serum tumor marker (CA15-3, CEA) monitoring.
Discussion
Analysis of circINTS4 expression across breast cancer subtypes revealed significantly elevated levels in HR+/HER2- (4.62-fold) and TNBC (4.26-fold) versus other subtypes. While expression differences relative to normal tissue (B-NML) lacked statistical significance (p > 0.05), comparable expression patterns in aggressive subtypes (TNBC and HR+/HER2-) suggest circINTS4 may regulate malignant pathways, consistent with established circRNA functions in tumor progression [6, 7]. Further validation studies are warranted.
Notably, circINTS4 expression was consistently lower in TNBC-NAT versus tumors, though statistically non-significant (p > 0.05). The limited sample size (n = 10 per group) reduces statistical power to detect true biological differences amid breast cancer’s pronounced heterogeneity. Despite non-significance, the biological relevance aligns with established tumor-normal circRNA disparities [8]. Future studies should: (1) employ larger cohorts to enhance detection sensitivity; (2) investigate spatial heterogeneity via in situ hybridization/single-cell sequencing; and (3) conduct clinicopathological subgroup analyses.
Specifically, HER2- tumors exhibited significantly elevated circINTS4 expression versus HER2+ controls (p < 0.05). This strong association suggests that circINTS4 may function through modulation of the HER2 signaling pathway. Previous studies have shown that overexpression of circCDYL promotes the progression of HER2- breast cancer through the miR-1275-ULK1/ATG7-autophagic axis [9]. We hypothesize that this elevated expression could represent a compensatory event worthy of further exploration, potentially offering new insights into the biology of HER2- disease. Further functional validation in larger cohorts is necessary to test this hypothesis.
CircINTS4 expression was lower in TNBC-NAT (TNBC-NAT: 0.17 vs. TNBC: 1.0, p = 0.13) with substantial effect size (Cohen’s d = 0.69), suggesting biological relevance. The TNBC > TNBC-NAT gradient may reflect tumor-induced epigenetic field effects on surrounding tissue, potentially preceding histopathological changes [10]. This supports future investigation of circINTS4 as a potential diagnostic biomarker for early TNBC, pending validation in expanded cohorts.
CircINTS4 expression showed no association with traditional clinicopathological parameters (size, nodal status, grade, LVI; all p > 0.05), contradicting conventional paradigms of invasion-driven molecular expression [11]. Its molecular subtype specificity (enriched in HER2-negative and TNBC) positions it as a potential new marker for refining subtype classification. While clinical translation requires further investigation, interdisciplinary approaches may position circINTS4 as a valuable breast cancer classifier and therapy response predictor [12], pending validation in expanded cohorts.
No recurrence occurred during postoperative follow-up (median: 22 months; range: 15–27) with standardized monitoring, suggesting circINTS4 expression may not influence short-term recurrence risk despite its potential tumorigenic role. Given established circRNA functions in metastasis [13] and peak breast cancer recurrence typically occurring at 2–3 years [14, 15], future large-scale longitudinal studies (≥ 200 cases, ≥ 5 years) should assess dynamic circINTS4 expression relationships with micrometastatic activation.
This study has limitations. The limited sample size restricts statistical power and generalizability, while the moderate follow-up precludes long-term outcome assessment. Our findings thus require validation in larger, longer-term cohorts.
In conclusion, circINTS4 demonstrates subtype-specific overexpression in HR+/HER2- and TNBC breast cancers, with significantly higher expression in HER2- versus HER2+ tumors (p < 0.05). It exhibits reduced expression in TNBC-adjacent tissues (TNBC-NAT) and shows no association with traditional clinicopathological parameters (tumor size, nodal status, grade, LVI) or short-term recurrence (≤ 27 months). These findings position circINTS4 as a candidate molecular classifier and early detection biomarker, warranting further investigation of its mechanistic roles and clinical utility.