The presence of vitamin D receptors in the human ovaries, uterus, and placenta has suggested its reproductive physiology role [1]. Infertile women with polycystic ovary syndrome (PCOS) have lower serum vitamin D than fertile women [2]. Women with endometriosis were found to have unbalanced, both higher [3] and lower [4], levels of vitamin D. In vitro fertilization (IVF) represents a valuable model for evaluating the influence of vitamin D on human fertility. It enables assessment of each stage of the reproductive process, from sperm function, through folliculogenesis, to embryo implantation [5]. Recently, follicular vitamin D levels are being considered as potential markers of the oocyte and embryo quality and predictors of IVF outcome [6].
Consequently, combining serum and follicular vitamin D analysis with embryo morphological assessment could provide a more accurate and sensitive method of determining embryonic developmental capability. Vitamin D has an effect on the outcome of controlled ovarian stimulation (COS) in women undergoing IVF [7, 8]. However, the correlation between serum and follicular fluid (FF) levels of vitamin D exclusively in women with unexplained infertility, identified in 10–30% of couples undergoing IVF, has not been investigated yet. Unexplained infertility is defined as the absence of conception despite 12 months of unprotected intercourse, not explained by anovulation, poor sperm quality, tubal pathology, or any known cause of infertility [9].
The main objective of this study was to determine correlations between serum and FF levels of vitamin D in women with unexplained infertility and to assess the relationship between serum and FF levels of vitamin D on the day of oocyte retrieval and some important IVF outcomes.
Methods
In this retrospective cross-sectional study, vitamin D levels in sera and FF of women undergoing IVF at the IVF Department of Clinic for Gynaecology and Obstetrics “Narodni front”, Belgrade, from September 2012 to January 2014 were measured and correlated with the study outcomes (e.g. the number of perifollicular follicles, the number of retrieved oocytes, the number of Metaphase II oocytes (MII), the percentage of embryo fragmentation, the two-pronuclear zygote (2pn), the number of embryos, and the implantation rate). The Institutional Review Board of the Clinic for Gynaecology and Obstetrics “Narodni front” approved the study (Decision#05006-2019-21822). Informed consent was provided by all participants.
Inclusion study criteria comprised unexplained infertility, age up to 38 years, body mass index (BMI) up to 30 kg/m2, first fresh autologous IVF cycle, and primary infertility. Exclusion criteria were known causes of male and female infertility (such as male infertility factor, endometriosis, and PCOS), factors related to female genitalia that could cause fertility problems (such as myomas distorting endometrial cavity, endometrial polyps, uterine anomalies), previous vitamin D supplementation, the use of drugs that affect the metabolism of vitamin D, earlier surgeries on the ovary and fallopian tubes, hypothalamic amenorrhoea, galactorrhoea, and systemic and infective diseases. Unexplained infertility was diagnosed after the absence of conception despite 12 months of unprotected intercourse, not explained by anovulation, poor sperm quality, tubal pathology, or any known cause of infertility such as thrombophilia, insulin resistance, thyroid disorders, etc. [9].
Practices and protocols of IVF Clinic for Gynaecology and Obstetrics “Narodni front” regarding COS, oocyte retrieval, and embryo transfer (ET) have been previously described [10–12]. Micronized progesterone 600 mg/day and 250 mg hydroxyprogesterone caproate were used for luteal support every fifth day. Serum β-hCG > 50 mIU/ml on the 16th day after retrieval was considered as biochemical pregnancy. A gestational sac or foetal pole visualized on an ultrasound at the 7th week of gestation was deemed as clinical pregnancy. All pregnancy rates were calculated per ET.
Blood was collected from all participants immediately before COS initiation. FF and blood samples were collected after COS on the day of oocyte retrieval. Serum oestradiol (E2) levels were measured by chemiluminescence assay using an automated analyser (Roche Diagnostics GmbH, Germany). Serum and FF vitamin D were measured by ELISA using 25-OH vitamin D-Euroimun from the Perkin Elmer company, Medizinische Labordiagnostika AG (Germany), using Multilabel Counter Perkin Elmer, Singapore.
The analysed characteristics of participants were as follows: BMI, infertility duration, baseline serum E2, antral follicle count (AFC). The analysed parameters of COS were as follows: perifollicular follicles, retrieved oocytes, and MII number, percentage of embryo fragmentation, 2pn, embryos number, and implantation rate. Perifollicular follicle identified by ultrasound at trigger day is defined as follicle with diameter > 16 mm. MII is an oocyte at metaphase II showing a polar body at the top. The percentage of embryo fragmentation presents a percentage of the perivitelline space’s volume and/or cleavage cavity occupied by anucleate cytoplasmic fragments. A 2pn is defined as a zygote with the appearance of two pronuclei observed 18 h after insemination/ICSI. The implantation rate (IR) is calculated as per equation: IR = n-gestational sacs/n-transferred embryos, where n-gestational sacs is the number of gestational sacs observed at vaginal ultrasound 3–5 weeks after transfer, and n-transferred embryos is the number of transferred embryos [13].
Statistical analysis
Descriptive statistical methods are used to present data as mean ± standard deviation (SD), median, and percentiles. Student’s t-test and Spearman’s correlation analysis were used as analytical, statistical methods. A p-value < 0.05 was considered significant. Statistical Package for the Social Sciences 12.0 (SPSS Inc., Chicago) was used for all statistical calculations.
Results
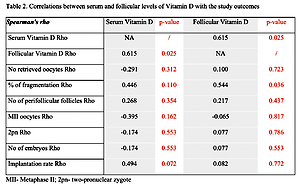
During the study period, 382 women underwent IVF procedures, of whom 107 were with unexplained infertility (the prevalence of unexplained infertility was 28.01%). The application of study inclusion/exclusion criteria left 16 patients to analyse further serum and FF vitamin D levels and parameters of IVF outcome. The mean age of the study cohort patients was 34.0 ±2.3 years, with a BMI of 22.11 ±1.62 kg/m2. The median duration of infertility was 4.6 ±3.5 years, and the AFC was 8.07 ±5.1, while the baseline serum E2 and AMH were 93.13 ±53.02 pmol/l and 2.51 ±1.93 ng/ml, respectively. Characteristics of IVF treatment/outcome and the serum and FF levels of vitamin D are presented in Table I. When compared, the vitamin D concentrations in serum and in FF showed no statistically significant differences (mean difference 4.6692, T 0.418, p = 0.684). A significant positive correlation was evident between serum and FF vitamin D levels according to Rho = 0.615, p = 0.025. The biochemical pregnancy rate was 56.25%, while the clinical pregnancy rate was 50%. All achieved pregnancies in the study population were singleton, and the live birth rate was 37.5%. Table II summarizes the results of the correlation analysis used to examine associations of serum and FF vitamin D levels with the study outcomes. A positive correlation between FF vitamin D levels and the percentage of embryo fragmentation (Rho = 0.544; p = 0.036) was determined. Other tested parameters of IVF outcome such as number of retrieved oocytes, number of perifollicular follicles, MII oocytes, 2pn, number of embryos, and implantation rate did not attain statistical significance with either serum or FF vitamin D levels.
Table I
Characteristics of IVF treatment and outcome and levels of vitamin D in serum and follicular fluid
Table II
Correlations between serum and follicular levels of vitamin D and the study outcomes
Discussion
Vitamin D receptors in the human ovaries, uterus, and placenta have a suggested reproductive physiology role [1]. Recently, follicular vitamin D levels have been considered as potential markers of the oocyte and embryo quality and predictors of IVF outcome [6]. Combining serum and follicular vitamin D analysis with embryo morphological assessment could provide a more accurate and sensitive method of determining embryonic developmental capability.
In the present study in women with unexplained infertility, we observed a significant positive correlation between serum and FF vitamin D levels. This finding is consistent with previous studies [6, 14–16], showing that the serum and FF vitamin D levels significantly correlate. We did not observe significant differences between serum and FF vitamin D concentrations. Contrary to our results, other studies show a higher vitamin D concentration in the FF than in serum [7, 14, 15]. The reason might be that the association between vitamin D and COS and IVF outcomes differs according to infertility diagnosis [7] in an overall population of infertile women undergoing IVF. Consequently, this issue has been evaluated in populations encompassing women with PCOS and endometriosis [2–4], known as women with altered vitamin D levels compared to the general female population. Also, the foundations of unexplained infertility evaluated in the current study might encompass elusive imbalances in a wide array, many of which may not be susceptible to the effects of serum and FF vitamin D levels.
With the regularity of blastomeres, the embryo fragmentation percentage is the most important in the embryo quality’s morphological assessment. It reflects not only the quality but also the viability of the embryo. The present study analysed the correlation between serum and FF vitamin D levels with the percentage of embryo fragmentation. The results obtained in this study for correlation between FF vitamin D and the percentage of embryo fragmentation are similar to the results reported in the other studies [6, 16]. This study shows a significant positive correlation between FF vitamin D and the percentage of embryo fragmentation. This result could mean that although vitamin D at physiological levels plays an important role in endometrial receptivity, its anti-oestrogenic effect is detrimental to oocyte development and embryo quality [17]. However, this issue should be considered differently in various infertility causes and in different metabolic and hormonal backgrounds.
In conclucion, the findings from this pilot study confirmed the feasibility of serum and FF vitamin D measurements in women with unexplained infertility undergoing IVF. Some of the results of this study suggest that combining serum and FF vitamin D measurements with other assessment tools (such as routine morphological assessment of the embryo) could allow a more precise method for predicting embryonic developmental competence and different important IVF outcomes. Although many factors can cause infertility, these findings are significant because vitamin D deficiency may explain some unexplained infertility cases or contribute to the other causes of infertility.
The limitations of the current study need to be reported. It is an observational pilot study with a small number of patients, and firm inclusion criteria were applied. Additionally, it has a retrospective design with different infertility assessments or different IVF approaches to infertile patients.
Although vitamin D seems to benefit the overall population of infertile women or those with PCOS or endometriosis, this influence has not yet been proven for women with unexplained infertility. Accordingly, it is crucial to test if facts recognized for the universal population are appropriate for each type of patient or infertility cause. Future prospective studies might be relevant, to advise whether it is reasonable to inform infertile women with unexplained infertility undergoing IVF to evaluate serum vitamin D status before COS initiation. Longitudinal studies with larger numbers of participants are necessary to confirm our results, i.e. that serum and follicular fluid vitamin D measurements could be complementary tools to the routine assessment of embryos.