Introduction
Extracorporeal life support (ECLS) is a technique to provide temporary support to cardiac or pulmonary function by using mechanical devices [1]. Extracorporeal life support is called extracorporeal membrane oxygenation (ECMO) when used in the intensive care unit (ICU) or emergency department (ED) setting to improve oxygenation, ventilation, or cardiac output [1]. Extracorporeal membrane oxygenation is used to bypass the cardiopulmonary system in patients with acute hypoxaemic respiratory failure, cardiac arrest, and cardiogenic shock, who are refractory to conventional intervention [1–3]. It can maintain tissue oxygenation for days to weeks in patients with respiratory or cardiac failure [4]. Recent studies have shown that ECMO might be a therapeutic option in refractory septic shock, myocarditis, and severe thoracic trauma patients [5–8]. There are 2 main forms of ECMO: veno-arterial (VA) ECMO and veno-venous (VV) ECMO. In VA ECMO, the circuit is connected in parallel to the heart and lungs whereas it is connected in series in VV ECMO [9]. Thus, VA ECMO provides cardiac support to assist systemic circulation whereas VV ECMO does not. A high mortality rate is associated with the use of either type of ECMO. As per the Extracorporeal Life Support Organisation (ELSO) registry report, only 43% of the adults who received ECMO for cardiac support and 59% for respiratory support survived to discharge or transfer [10]. In a meta-analysis on the outcomes of ECMO, Zangrillo et al. noted an overall mortality of 54% at 30 days post ECMO [11]. Haemorrhage is the most frequent complication during ECMO [9]. Bleeding may occur at the cannulation site, or it may be intracerebral, intrathoracic, gastrointestinal, or retroperitoneal [12]. Gastrointestinal bleeding (GIB) has been shown to be associated with increased mortality in ECMO patients in a number of studies [13–15]. However, epidemiological data on the outcomes and economic burden of gastrointestinal haemorrhage (GIH) associated with ECMO hospitalisations are limited.
Material and methods
Source of data
The NIS, designed by the Agency for Healthcare Research and Quality (AHRQ), is the largest all-payer inpatient database in the U.S. Each individual hospitalisation is de-identified and maintained in the NIS as a unique entry with one primary discharge diagnosis and up to 24 secondary diagnoses during that hospitalisation. Each entry includes demographic information including race, age, primary payer, income level by zip code as well as medical comorbidities, primary/secondary procedures, hospitalisation outcomes, length of stay, and cost of care.
Data are compiled yearly and contain discharge information from over 1200 hospitals located across 45 states in the U.S. The NIS was designed to approximate a 20% stratified sample of community hospitals in the country and provides sampling weights to calculate national estimates [16]. The internal validity of the database is guaranteed by annual data quality assessments of the sample. Moreover, comparisons with data sources like the American Hospital Association (AHA) Annual Survey of Hospitals, National Hospital Discharge Survey from the National Centre for Health Statistics, and Medicare Provider and Analysis Review (MedPAR) inpatient data from the Centres for Medicare and Medicaid Services strengthen the external validity of the sample [17, 18]. It contains information included in a typical discharge summary, with safeguards in place to protect the privacy of individual patients, physicians, and hospitals.
Study design
This is a retrospective cohort study in which we queried the NIS database from the year 2007 to 2011 to identify all the hospitalisations with ECMO using ICD-9 code 39.65. We then extracted data for all hospitalisations with GIH from this cohort by using the appropriate ICD-9 codes (456.x, 530.x, 531.x, 532.x, 533.x, 534.x, 569.x, and 578.x). Hospitalisations for patients less than 18 years of age and those missing demographic information or admission/discharge date, in-hospital mortality status and demographics and comorbidities were excluded [19, 20]. NIS data were merged with cost-to-charge ratio (CCR) files available from the Healthcare Cost and Utilisation Project (HCUP) to estimate cost of hospitalisations.
Variables and statistical analysis
Analyses were performed using hospital-level discharge weights provided by the NIS, to obtain national estimates of hospitalizations. The Cochrane-Armitage trend test was used to calculate trends in categorical variables [21]. The Wilcoxon rank sum test was used to assess continuous variables [22]. A p-value less than 0.05 was considered significant. Hospitalisations were also stratified by subgroups of age (18–34, 35–49, 50–64, 65–79, and > 80 years), gender, race (White, Black, Hispanic, and Others), insurance status (Medicare/Medicaid, private insurance, and self-pay/other), hospital location in different U.S. regions (Northeast, Midwest, South, and West), bed size of the hospital (small, medium, and large), and teaching status of the hospital (urban teaching, urban non-teaching, and rural). In keeping with AHRQ definitions, a hospital was considered to be a teaching hospital if it is: a) an AMA-approved residency program, b) a member of the Council of Teaching Hospitals, or c) a hospital with a full-time intern and resident-to-bed (IRB) ratio more than 0.25 [23]. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Demographics and trends in hospitalisations
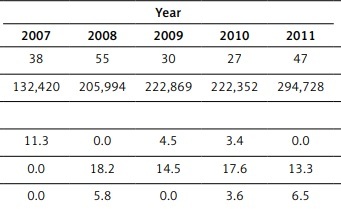
Patient characteristics are summarised in Table I. The number of hospitalisations with a diagnosis code for ECMO increased progressively from 1869 in 2007 to 3799 in 2011 (p < 0.01). During the study period, 14,793 hospitalisations with ECMO were recorded. Of these, 659 hospitalisations (4.5%) were found to have GIH. The percentage of hospitalisations with GIH associated with ECMO increased from 2.1% in 2007 to 7.5% in 2011 (p < 0.01). The majority of patients were white (57.4%) males (56.7%) between the ages of 18 and 34 years (23.5%). The highest rates of ECMO-related hospitalisations were observed in the southern region (36.2%), and the lowest number were seen in western region (17.7%). The vast majority of the hospitalisations were seen in urban teaching hospitals (91.6%). Hospitalisations with GIH associated with ECMO progressively increased in urban teaching hospitals from 88.9% in 2007 to 93.1% in 2011 (p < 0.01), and those in urban non-teaching decreased from 11.1% in 2007 to 3.3% in 2011 (p < 0.01). Private insurance paid for 39.1% and Medicaid paid for 28.1% of the total hospitalisations studied.
Table I
Baseline characteristics of patients with gastrointestinal haemorrhage (GIH) during extracorporeal membrane oxygenation (ECMO) hospitalisations
[i] AHRQ – Agency for Healthcare Research and Quality. *Variables are AHRQ comorbidity measures. ^Neurological disorders include hemiplegia, paralysis, and others. ~Psychiatric disorders include depression, psychosis, and others. $Rheumatic disorders include rheumatoid arthritis and other collagen vascular disorders.
Length of stay and cost of care
Median length of stay (LOS) was 38 days (interquartile range 14 to 52 days). The mean cost of care for hospitalisation with GIB associated with ECMO increased significantly from $132,420 in 2007 to $294,728 in 2011 (p < 0.01).
All-cause inpatient mortality
The overall in-hospital all-cause mortality associated with GIH in ECMO hospitalisations was 59.3% (Table II). There was an insignificant rise from 56.7% in 2007 to 61.9% in 2011 (p = 0.49). The mortality rate was highest in the age group over 80 years (100%) and lowest in the 35–49-year-old age group (58.1%). The mortality rate was lowest for whites (52.5%); however, the percentage increase in the white population was highest between 2007 and 2011 (163%). It was higher in males (62.4%) and blacks (72.1%).
Table II
In-hospital mortality rate in patients with GIH during ECMO hospitalisations (%)
| Parameter | Year | Overall | Percentage change | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | ||||
| Overall | 56.7 | 64.1 | 61.5 | 50.8 | 61.9 | 59.3 | 9.2 | 0.49 |
| Age [years] (%): | ||||||||
| 18–34 | 65.4 | 100.0 | 37.6 | 100.0 | 72.5 | 71.4 | 10.9 | 0.09 |
| 35–49 | 0.0 | 67.6 | 77.3 | 50.3 | 56.9 | 58.1 | 56.9 | 0.01 |
| 50–64 | 0.0 | 100.0 | 100.0 | 67.4 | 65.6 | 71.3 | 65.6 | < 0.01 |
| 65–79 | 0.0 | 75.6 | 50.0 | 50.6 | 71.1 | 62.9 | 71.1 | 0.22 |
| ≥ 80 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100 | 100.0 | 0.04 |
| Gender (%): | ||||||||
| Male | 48.0 | 69.4 | 71.6 | 53.5 | 65.7 | 62.4 | 36.9 | 0.01 |
| Female | 75.0 | 56.6 | 52.5 | 45.2 | 57.8 | 55.3 | –22.9 | 0.03 |
| Race (%): | ||||||||
| White | 21.0 | 66.6 | 54.6 | 45.1 | 55.2 | 52.5 | 163.1 | < 0.01 |
| Black | 0.0 | 100.0 | 50.6 | 76.3 | 71.2 | 72.1 | 71.2 | 0.01 |
| Hispanic | 65.4 | 100.0 | 100.0 | 33.0 | 76.6 | 71.3 | 17.0 | 0.02 |
| Others | 100.0 | 34.4 | 100.0 | 100.0 | 100.0 | 81.0 | 0.0 | * |
| Region (%): | ||||||||
| Northeast | 0.0 | 60.0 | 100.0 | 83.3 | 55.6 | 67.9 | 55.6 | < 0.01 |
| Midwest | 100.0 | 0.0 | 57.2 | 16.4 | 80.0 | 58.3 | –20.0 | 0.39 |
| South | 61.7 | 83.7 | 57.9 | 61.3 | 62.5 | 64.3 | 1.3 | 0.03 |
| West | 33.8 | 58.6 | 38.6 | 33.5 | 26.3 | 38.7 | –22.3 | < 0.01 |
| Location (%): | ||||||||
| Rural | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | 100.0 | 0.04 |
| Urban nonteaching | 0.0 | 100.0 | 46.6 | 0.0 | 0.0 | 22.5 | * | < 0.01 |
| Urban teaching | 63.8 | 61.6 | 63.1 | 56.9 | 62.6 | 61.4 | –1.8 | < 0.01 |
| Median household income (%): | ||||||||
| Quartile 1 | 0.0 | 0.0 | 42.4 | 63.1 | 61.1 | 52.5 | 61.1 | 0.04 |
| Quartile 2 | 100.0 | 100.0 | 73.8 | 37.6 | 38.3 | 55.7 | –61.7 | 0.22 |
| Quartile 3 | 50.0 | 55.3 | 50.3 | 61.4 | 75.6 | 64.1 | 51.3 | 0.05 |
| Quartile 4 | 52.0 | 100.0 | 75.2 | 34.7 | 65.3 | 61.9 | 25.7 | 0.16 |
| Payment (%): | ||||||||
| Medicare | 0.0 | 80.3 | 76.2 | 34.1 | 79.8 | 63.7 | 79.8 | 0.12 |
| Medicaid | 65.4 | 47.5 | 49.0 | 40.8 | 32.6 | 41.7 | –50.1 | < 0.01 |
| Private insurance | 50.3 | 56.8 | 55.7 | 86.2 | 70.4 | 67.0 | 39.8 | < 0.01 |
| Others (includes self-pay) | 53.6 | 100.0 | 100.0 | 49.8 | 65.7 | 68.1 | 22.5 | 0.05 |
AHRQ co-morbidities
As per our analysis, the most frequent coexisting conditions coded with these hospitalisations were renal failure (70.9%), anaemia (54.5%), and congestive heart failure (26.8%). Several other comorbidities were also associated, as seen in Table I.
Discussion
Extracorporeal membrane oxygenation use has been increasing over the past years. Fenton et al. found that unweighted ECMO discharges increased from 352 in 2002 to 2715 in 2012 [1]. Our analysis shows an increase in ECMO-related hospitalisations from 1869 in 2007 to 3799 in 2011 (p < 0.01). ECMO use is associated with multiple complications, of which bleeding and thrombosis are the major determinants of mortality [11, 13, 24–28]. We identified an increasing proportion of hospitalisations for ECMO which were associated with coagulopathy and GIH.
In earlier reports, GIH was noted in less than 10% of patients [29–31]. In a retrospective cohort study conducted by Mazzeffi et al. 18 out of 132 recruited ECMO patients (13.6%) had GIB [26]. In a similar study conducted at Westchester Medical Centre in patients with VA ECMO and concomitant IABP implantation, 21 out of 135 patients developed GIB (15.6%) [32]. These numbers are higher, compared to the rate noted in our study (4.5%). This may be a result of possible differences in the characteristics of the cohorts studied, comorbid conditions, sample size, and coding inaccuracies.
Sepsis, multi-organ failure, and imbalance between pro-coagulant and anticoagulant pathways cause platelet activation and consumption of clotting factors, leading to an acquired state of clotting factor deficiency [33–35]. Contact between blood and non-endothelial surface activates the coagulation pathway leading to thrombosis and subsequent bleeding [34]. Utilisation of large bore arterial and venous access also lead to bleeding complications [34]. Because heparin is widely used for anticoagulation in these patients, heparin-induced thrombocytopaenia (HIT) may also be a major factor responsible for bleeding. GIB in ECMO patients is also attributed to arterio-venous malformation (AVM), which is stimulated by tissue hypoperfusion caused by non-pulsatile flow [36, 37]. These factors play an important role in promoting GIH in patients undergoing ECMO, while other factors not yet identified may contribute to GIH as well.
Mazzeffi et al. achieved limited success in preventing GIH in ECMO patients with the use of prophylactic measures such as proton pump inhibitor (PPI) therapy [14]. In many cases, despite investigation by upper and lower endoscopy and tagged red blood cell (RBC) scans, no discrete bleeding source is identified in these patients [14]. However, it is crucial to note that the sample size of this study was small. In largescale multi-centre trials, PPIs might show better outcomes.
Throughout our study period, in-hospital mortality was more than 50%, which has not changed significantly in the last few years. With the increasing use of newer interventions, especially membrane oxygenators, bleeding and thrombotic complication associated with ECMO use may be reduced [38]. VV ECMO use in select patients rather than VA ECMO could also reduce incidence of GI haemorrhage and haemolysis, leading to better survival and lesser complications [30].
Our results also demonstrated higher overall mortality rates among black males. A similar finding was made in a single-centre study of patients who had received VV ECMO (n = 41), in which members of minority groups (n = 11) had increased mortality at 30 days and 1 year post ECMO [32]. Race was noted to be an independent predictor for survival at 30 days, although this was no longer statistically significant after adjustment for shock and lung transplant status. Limited data are available to compare hospitalisations by geographic region. In the present study, we found that ECMO hospitalisations in the south were most common and were least common in the west. In the past 2 years, there has been an increasing trend toward hospitalisations in the northeast and mid-west. According to a study in Norway, more than 90% of all ECMO therapy was performed in urban teaching hospitals, while only around 2% was performed in rural hospitals [39]. In our study, close to 92% of all the hospitalisations were seen in urban teaching hospitals. We observed a 62.8% increase in the average cost for hospitalisation for GIB associated with ECMO use. Such a trend was previously noted in an earlier analysis by Maxwell et al., which found that for ECMO hospitalisations between 1998 and 2009, there was an increase in hospital costs and worse outcomes, in large measure related to use of ECMO in non-cardiotomy patients [40]. The present results are consistent with this trend and also may relate to increasing complexity of care and use of ECMO among patients with more serious medical comorbidities.
This study identifies gastrointestinal bleeding as a major complication in patients receiving ECMO therapy, which puts considerable economic burden on the healthcare system. Our study results have important implications because they are applicable to the patients receiving acute interventional care for life-threatening clinical conditions in hospitals all over the U.S. The causes and mechanisms leading to GIB in these patients are not yet fully understood. In the future, animal models might help us understand the exact pathophysiological basis of gastrointestinal bleeding in these patients. Randomised clinical trials (RCTs) comparing ECMO with conventional therapies might identify the risk factors associated with GIB in ECMO patients. For now, we believe that stringent protocols for initiation and maintenance of anti-coagulation in ECMO patients can reduce the incidence of GIB. We also believe that PPIs remain crucial in preventing upper GIB in these patients.
This study has several limitations. Firstly, the NIS does not contain data related to certain aspects of clinical management. Variability in the use and impact of modalities employed to mitigate against bleeding could therefore not be accounted for in this study. Because of the lack of specificity in codes identifying GIH, the particular aetiology of haemorrhage could also not be determined. Furthermore, this analysis could not account for the duration of ECMO, which has a direct association with bleeding complications [41]. It also could not ascertain whether VV or VA ECMO was utilised and how that may have influenced the outcomes.
In conclusion, there was a significant increase in ECMO hospitalisations with gastrointestinal bleeding during our study period. Also, the inpatient mortality rate and cost of care increased significantly in ECMO hospitalisations with gastrointestinal bleeding during the study period.