Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / BASIC RESEARCH
KDM1A represses apoptosis in osteosarcoma cells via demethylating Bcl-2/c-Myc
1
Department of Orthopedics Surgery, Xixi Hospital of Hangzhou, Hangzhou, Zhejiang Province, China
2
Department of Orthopedics Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China
3
Orthopedics Research Institute of Zhejiang University, Hangzhou, Zhejiang Province, China
4
Key Laboratory of Motor System Disease Research and Precision Therapy of Zhejiang Province, Hangzhou, Zhejiang Province, China
Submission date: 2023-03-17
Final revision date: 2023-07-17
Acceptance date: 2023-07-22
Online publication date: 2023-08-07
Corresponding author
Wei Gao
Department of Orthopedics Surgery, Xixi Hospital of Hangzhou, No. 2 Hengbu Street, Liuxia town, Xihu District, Hangzhou 310012, Zhejiang Province, China
Department of Orthopedics Surgery, Xixi Hospital of Hangzhou, No. 2 Hengbu Street, Liuxia town, Xihu District, Hangzhou 310012, Zhejiang Province, China
KEYWORDS
osteosarcomalysine demethylase 1Aapoptosisreactive oxygen speciesendoplasmic reticulum stressdemethylationBcl-2/c-Myc
TOPICS
ABSTRACT
Introduction:
Considering the poorly understood role of lysine demethylase 1A (KDM1A) in osteosarcoma (OS), we conducted this study to elucidate the underlying mechanism.
Material and methods:
Following the appropriate transfection, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) and flow cytometry assays were used to determine the viability and apoptosis of MG-63 OS cells, in which the generation of reactive oxygen species (ROS) and the binding between KDM1A and Bcl-2/ cellular Myc (c-Myc) were separately confirmed via the DCF-DA (2,7-dichlorodihydrofluorescein diacetate) method and chromatin immunoprecipitation-PCR. Reverse-transcription quantitative PCR and western blot were finally introduced to quantify the levels of KDM1A/Bcl-2/c-Myc and endoplasmic reticulum (ER) stress-related factors.
Results:
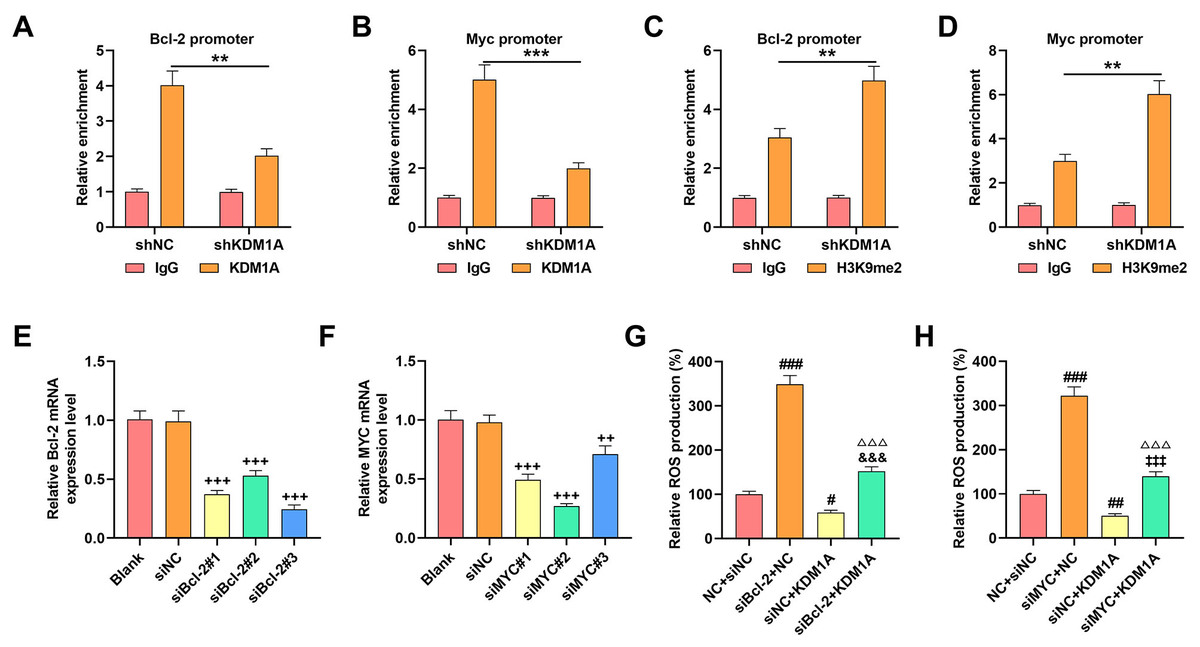
Overexpressed KDM1A enhanced the viability (48 h) yet repressed the apoptosis and ROS generation, with downregulation of ER stress-related factors (C/EBP homologous protein [CHOP]; proline-rich extensin-like receptor kinase (PERK) and activating transcription factor 4 [ATF4]) but elevation of Bcl-2/c-Myc, while its depletion exerted contrary effects. More importantly, KDM1A could act as the demethylase of Bcl-2/c-Myc, as reflected by the results showing that the depletion of KDM1A decreased the enrichment of Bcl-2/c-Myc promoter using the antibody against KDM1A yet increased the enrichment by the antibody targeting H3K9me2. Bcl-2/c-Myc silencing, conversely, promoted ROS generation and apoptosis, elevated the levels of ER stress-related factors and abolished the effects of KDM1A on OS cells.
Conclusions:
KDM1A exerts a repressive effect on the apoptosis of MG-63 OS cells by inhibiting ROS generation and ER stress via demethylation of Bcl-2 and c-Myc.
Considering the poorly understood role of lysine demethylase 1A (KDM1A) in osteosarcoma (OS), we conducted this study to elucidate the underlying mechanism.
Material and methods:
Following the appropriate transfection, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) and flow cytometry assays were used to determine the viability and apoptosis of MG-63 OS cells, in which the generation of reactive oxygen species (ROS) and the binding between KDM1A and Bcl-2/ cellular Myc (c-Myc) were separately confirmed via the DCF-DA (2,7-dichlorodihydrofluorescein diacetate) method and chromatin immunoprecipitation-PCR. Reverse-transcription quantitative PCR and western blot were finally introduced to quantify the levels of KDM1A/Bcl-2/c-Myc and endoplasmic reticulum (ER) stress-related factors.
Results:
Overexpressed KDM1A enhanced the viability (48 h) yet repressed the apoptosis and ROS generation, with downregulation of ER stress-related factors (C/EBP homologous protein [CHOP]; proline-rich extensin-like receptor kinase (PERK) and activating transcription factor 4 [ATF4]) but elevation of Bcl-2/c-Myc, while its depletion exerted contrary effects. More importantly, KDM1A could act as the demethylase of Bcl-2/c-Myc, as reflected by the results showing that the depletion of KDM1A decreased the enrichment of Bcl-2/c-Myc promoter using the antibody against KDM1A yet increased the enrichment by the antibody targeting H3K9me2. Bcl-2/c-Myc silencing, conversely, promoted ROS generation and apoptosis, elevated the levels of ER stress-related factors and abolished the effects of KDM1A on OS cells.
Conclusions:
KDM1A exerts a repressive effect on the apoptosis of MG-63 OS cells by inhibiting ROS generation and ER stress via demethylation of Bcl-2 and c-Myc.
REFERENCES (56)
1.
Eaton BR, Schwarz R, Vatner R, et al. Osteosarcoma. Pediatr Blood Cancer 2021; 68 Suppl 2: e28352.
2.
Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett 2021; 500: 1-10.
3.
Yoshida A. Osteosarcoma: old and new challenges. Surg Pathol Clin 2021; 14: 567-83.
4.
Ghafouri-Fard S, Shirvani-Farsani Z, Hussen BM, Taheri M. The critical roles of lncRNAs in the development of osteosarcoma. Biomed Pharmacother 2021; 135: 111217.
5.
Chen Y, Liu R, Wang W, et al. Advances in targeted therapy for osteosarcoma based on molecular classification. Pharmacol Res 2021; 169: 105684.
6.
Ozdek A, Sarac S, Akyol MU, Sungur A, Yilmaz T. c-myc and bcl-2 Expression in supraglottic squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg 2004; 131: 77-83.
7.
Wu X, Cai ZD, Lou LM, Zhu YB. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol 2012; 36: 212-6.
8.
Du X, Fu X, Yao K, et al. Bcl-2 delays cell cycle through mitochondrial ATP and ROS. Cell Cycle 2017; 16: 707-13.
9.
Yang S, Zhou F, Dong Y, Ren F. -Mangostin induces apoptosis in human osteosarcoma cells through ROS-mediated endoplasmic reticulum stress via the WNT pathway. Cell Transplant 2021; 30: 9636897211035080.
10.
DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475: 106-9.
11.
Feng W, Dean DC, Hornicek FJ, et al. Myc is a prognostic biomarker and potential therapeutic target in osteosarcoma. Ther Adv Med Oncol 2020; 12: 1758835920922055.
12.
Asano N, Takeshima H, Yamashita S, et al. Epigenetic reprogramming underlies efficacy of DNA demethylation therapy in osteosarcomas. Sci Rep 2019; 9: 20360.
13.
Xu J, Li D, Cai Z, et al. An integrative analysis of DNA methylation in osteosarcoma. J Bone Oncol 2017; 9: 34-40.
14.
Chen XG, Ma L, Xu JX. Abnormal DNA methylation may contribute to the progression of osteosarcoma. Mol Med Rep 2018; 17: 193-9.
15.
Perillo B, Tramontano A, Pezone A, Migliaccio A. LSD1: more than demethylation of histone lysine residues. Exp Mol Med 2020; 52: 1936-47.
16.
Gu F, Lin Y, Wang Z, et al. Biological roles of LSD1 beyond its demethylase activity. Cell Mol Life Sci 2020; 77: 3341-50.
17.
Shao G, Wan X, Lai W, et al. Inhibition of lysine-specific demethylase 1 prevents proliferation and mediates cisplatin sensitivity in ovarian cancer cells. Oncol Lett 2018; 15: 9025-32.
18.
Bennani-Baiti IM, Machado I, Llombart-Bosch A, Kovar H. Lysine-specific demethylase 1 (LSD1/KDM1A/AOF2/BHC110) is expressed and is an epigenetic drug target in chondrosarcoma, Ewing’s sarcoma, osteosarcoma, and rhabdomyosarcoma. Human Pathol 2012; 43: 1300-7.
19.
Miao Y, Liu G, Liu L. Histone methyltransferase SUV39H2 regulates LSD1-dependent CDH1 expression and promotes epithelial mesenchymal transition of osteosarcoma. Cancer Cell Int 2021; 21: 2.
20.
Mikulčić M, Tabrizi-Wizsy NG, Bernhart EM, et al. 15d-PGJ(2) promotes ROS-dependent activation of MAPK-induced early apoptosis in osteosarcoma cell in vitro and in an ex ovo CAM assay. Int J Mol Sci 2021; 22: 11760.
21.
Hou X, Li Q, Yang L, et al. KDM1A and KDM3A promote tumor growth by upregulating cell cycle-associated genes in pancreatic cancer. Exp Biol Med 2021; 246: 1869-83.
22.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402-8.
23.
Shimizu T, Kamel WA, Yamaguchi-Iwai S, Fukuchi Y, Muto A, Saya H. Calcitriol exerts an anti-tumor effect in osteosarcoma by inducing the endoplasmic reticulum stress response. Cancer Sci 2017; 108: 1793-802.
24.
Sun X, Gu X, Li H, et al. H3K9me2 regulates early transcription factors to promote mesenchymal stem cell differentiation into cardiomyocytes. Mol Med Rep 2021; 24: 616.
25.
Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J Hematol Oncol 2019; 12: 129.
26.
Ambrosio S, Saccà CD, Majello B. Epigenetic regulation of epithelial to mesenchymal transition by the Lysine-specific demethylase LSD1/KDM1A. Biochim Biophys Acta Gene Regul Mech 2017; 1860: 905-10.
27.
Karakaidos P, Verigos J, Magklara A. LSD1/KDM1A, a gate-keeper of cancer stemness and a promising therapeutic target. Cancers 2019; 11: 1821.
28.
Huang M, Chen C, Geng J, et al. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett 2017; 398: 12-21.
29.
Wan Y, Yang L, Jiang S, Qian D, Duan J. Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm Bowel Dis 2022; 28: 639-48.
30.
Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000; 5: 415-8.
31.
Zhuang C, Ni S, Yang ZC, Liu RP. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of angelica sinensis polysaccharide. Oxid Med Cell Longev 2020; 2020: 3240820.
32.
Rowe LA, Degtyareva N, Doetsch PW. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radical Biol Med 2008; 45: 1167-77.
33.
Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett 2017; 387: 95-105.
34.
Lin H, Peng Y, Li J, et al. Reactive oxygen species regulate endoplasmic reticulum stress and ER-mitochondrial Ca(2+) crosstalk to promote programmed necrosis of rat nucleus pulposus cells under compression. Oxid Med Cell Longev 2021; 2021: 8810698.
35.
Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 2016; 17: 327.
36.
Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One 2007; 2: e835.
37.
Hu H, Tian M, Ding C, Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 2018; 9: 3083.
38.
Wortel IMN, van der Meer LT, Kilberg MS, van Leeuwen FN. Surviving stress: modulation of ATF4-mediated stress responses in normal and malignant cells. Trends Endocrinol Metab 2017; 28: 794-806.
39.
B’Chir W, Maurin AC, Carraro V, et al. The eIF2/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 2013; 41: 7683-99.
40.
van Vliet AR, Giordano F, Gerlo S, et al. The ER stress sensor PERK coordinates ER-plasma membrane contact site formation through interaction with filamin-A and F-actin remodeling. Mol Cell 2017; 65: 885-99.
41.
Dadey DYA, Kapoor V, Khudanyan A, et al. PERK regulates glioblastoma sensitivity to ER stress although promoting radiation resistance. Mol Cancer Res 2018; 16: 1447-53.
42.
Rivers A, Jagadeeswaran R, Lavelle D. Potential role of LSD1 inhibitors in the treatment of sickle cell disease: a review of preclinical animal model data. Am J Physiol Regul Integr Comp Physiol 2018; 315: R840-r7.
43.
Pishas KI, Drenberg CD, Taslim C, et al. Therapeutic targeting of KDM1A/LSD1 in Ewing sarcoma with SP-2509 engages the endoplasmic reticulum stress response. Mol Cancer Ther 2018; 17: 1902-16.
44.
Wang S, Li H, Chen S, et al. Andrographolide induces apoptosis in human osteosarcoma cells via the ROS/JNK pathway. Int J Oncol 2020; 56: 1417-28.
45.
Wang GS, Chen JY, Chen WC, et al. Surfactin induces ER stress-mediated apoptosis via IRE1-ASK1-JNK signaling in human osteosarcoma. Environ Toxicol 2022; 37: 574-84.
46.
Bochtler M, Kolano A, Xu GL. DNA demethylation pathways: additional players and regulators. BioEssays 2017; 39: 1-13.
47.
Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 2010; 11: 607-20.
48.
Neja SA. Site-specific DNA demethylation as a potential target for cancer epigenetic therapy. Epigenet Insights 2020; 13: 2516865720964808.
49.
Wang F, Qin G, Liu J, Wang X, Ye B. Integrated genome-wide methylation and expression analyses reveal key regulators in osteosarcoma. Comput Mathem Methods Med 2020; 2020: 7067649.
50.
Perillo B, Ombra MN, Bertoni A, et al. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 2008; 319: 202-6.
51.
Wissmann M, Yin N, Müller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 2007; 9: 347-53.
52.
Wang J, Hevi S, Kurash JK, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet 2009; 41: 125-9.
53.
Su CH, Tzeng TY, Cheng C, Hsu MT. An H2A histone isotype regulates estrogen receptor target genes by mediating enhancer-promoter-3’-UTR interactions in breast cancer cells. Nucleic Acids Res 2014; 42: 3073-88.
54.
Fairlie WD, Lee EF. Co-operativity between MYC and BCL-2 pro-survival proteins in cancer. Int J Mol Sci 2021; 22: 2841.
55.
Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene 2004; 23: 2797-808.
56.
Mosammaparast N, Kim H, Laurent B, et al. The histone demethylase LSD1/KDM1A promotes the DNA damage response. J Cell Biol 2013; 203: 457-70.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.