Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / CLINICAL RESEARCH
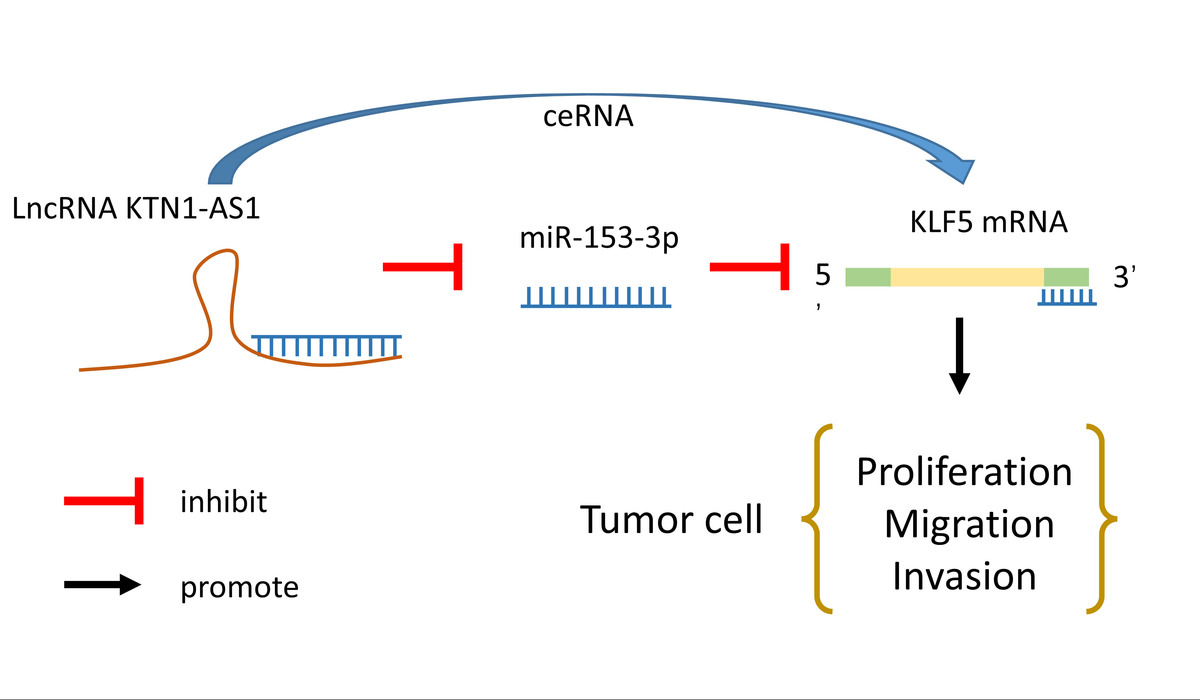
LncRNA KTN1-AS1 drives tumor progression in non-small cell lung cancer through the microRNA-153-3p/KLF5 axis
1
Department of Medical Oncology, Shaoxing People’s Hospital, Zhejiang, China
2
Alberta Institute, Wenzhou Medical University, Zhejiang, China
Submission date: 2023-09-20
Final revision date: 2024-01-24
Acceptance date: 2024-04-14
Online publication date: 2024-04-28
Corresponding author
KEYWORDS
KTN1-AS1 drove proliferationmigrationand invasion of NSCLC cells by regulating the microRNA-153-3p/KLF5 axis
TOPICS
ABSTRACT
Introduction:
The most typical kind of lung cancer is non-small cell lung cancer (NSCLC). Surgery, targeted therapy, chemotherapy, and immunotherapy are all options for the treatment of NSCLC. LncRNA KTN1-AS1 is significantly elevated in NSCLC, and it modulates the expression of microRNAs and downstream genes, promoting NSCLC progression.
Material and methods:
TCGA was employed to predict lncRNA KTN1-AS1 expression in NSCLC. The starBase database was utilized to predict downstream microRNAs of KTN1-AS1. The RNA22 database was employed to predict corresponding binding sites. Subcellular localization of KTN1-AS1 was forecasted using the lncLocator database, and the results were validated by FISH. qRT-PCR was used to test KTN1-AS1, microRNA-153-3p, and KLF5 expression. CCK-8, flow cytometry, and Transwell assay were used to determine cell viability, proliferation, migration, and invasion. Western blot was used to test KLF5 and Ki67 protein levels, and dual-luciferase assay was used to assess binding of KTN1-AS1 with microRNA-153-3p, and KLF5 with microRNA-153-3p.
Results:
KTN1-AS1 was significantly upregulated in NSCLC cells. Silencing KTN1-AS1 significantly repressed the proliferation, migration, and invasion of NSCLC cells. KTN1-AS1 bound to microRNA-153-3p, and KLF5 was a direct target of microRNA-153-3p. Inhibition of microRNA-153-3p or overexpression of KLF5 restored the stimulatory impact of KTN1-AS1 knockdown on NSCLC cell proliferation and migration.
Conclusions:
KTN1-AS1 drove proliferation, migration, and invasion of NSCLC cells by regulating the microRNA-153-3p/KLF5 axis.
The most typical kind of lung cancer is non-small cell lung cancer (NSCLC). Surgery, targeted therapy, chemotherapy, and immunotherapy are all options for the treatment of NSCLC. LncRNA KTN1-AS1 is significantly elevated in NSCLC, and it modulates the expression of microRNAs and downstream genes, promoting NSCLC progression.
Material and methods:
TCGA was employed to predict lncRNA KTN1-AS1 expression in NSCLC. The starBase database was utilized to predict downstream microRNAs of KTN1-AS1. The RNA22 database was employed to predict corresponding binding sites. Subcellular localization of KTN1-AS1 was forecasted using the lncLocator database, and the results were validated by FISH. qRT-PCR was used to test KTN1-AS1, microRNA-153-3p, and KLF5 expression. CCK-8, flow cytometry, and Transwell assay were used to determine cell viability, proliferation, migration, and invasion. Western blot was used to test KLF5 and Ki67 protein levels, and dual-luciferase assay was used to assess binding of KTN1-AS1 with microRNA-153-3p, and KLF5 with microRNA-153-3p.
Results:
KTN1-AS1 was significantly upregulated in NSCLC cells. Silencing KTN1-AS1 significantly repressed the proliferation, migration, and invasion of NSCLC cells. KTN1-AS1 bound to microRNA-153-3p, and KLF5 was a direct target of microRNA-153-3p. Inhibition of microRNA-153-3p or overexpression of KLF5 restored the stimulatory impact of KTN1-AS1 knockdown on NSCLC cell proliferation and migration.
Conclusions:
KTN1-AS1 drove proliferation, migration, and invasion of NSCLC cells by regulating the microRNA-153-3p/KLF5 axis.
REFERENCES (27)
1.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7-33.
2.
Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of non-small-cell lung cancer. N Engl J Med 2017; 376: 2109-21.
3.
Lin W, Zhou Q, Wang CQ, et al. LncRNAs regulate metabolism in cancer. Int J Biol Sci 2020; 16: 1194-206.
4.
Bautista RR, Gomez AO, Miranda AH, et al. Correction to: Long non-coding RNAs: implications in targeted diagnoses, prognosis, and improved therapeutic strategies in human non- and triple-negative breast cancer. Clin Epigenetics 2018; 10: 106.
5.
Xie X, Wen Q, Yang X, et al. H3K27ac-activated lncRNA KTN1-AS1 aggravates tumor progression by miR-505-3p/ZNF326 axis in ovarian cancer. Ann Transl Med 2022; 10: 599.
6.
Li C, Zhao W, Pan X, et al. LncRNA KTN1-AS1 promotes the progression of non-small cell lung cancer via sponging of miR-130a-5p and activation of PDPK1. Oncogene 2020; 39: 6157-71.
7.
Liu C, Li X, Hao Y, et al. STAT1-induced upregulation of lncRNA KTN1-AS1 predicts poor prognosis and facilitates non-small cell lung cancer progression via miR-23b/DEPDC1 axis. Aging (Albany NY) 2020; 12: 8680-701.
8.
Zhou H, Chang J, Zhang J, et al. PRMT5 activates KLF5 by methylation to facilitate lung cancer. J Cell Mol Med 2024; 28: e17856.
9.
Hu YP, Jin YP, Wu XS, et al. LncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Mol Cancer 2019; 18: 167.
10.
Cao P, Li F, Xiao Y, et al. Identification and validation of 7-lncRNA signature of epigenetic disorders by comprehensive epigenetic analysis. Dis Markers 2022; 2022: 5118444.
11.
Zhao L, Bi M, Zhang H, Shi M. Downregulation of NEAT1 suppresses cell proliferation, migration, and invasion in NSCLC via sponging miR-153-3p. Cancer Biother Radiopharm 2020; 35: 362-70.
12.
Luo Y, Chen C. The roles and regulation of the KLF5 transcription factor in cancers. Cancer Sci 2021; 112: 2097-117.
13.
Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol 2021; 16: 205-15.
14.
Zhang G, Li S, Lu J, et al. LncRNA MT1JP functions as a ceRNA in regulating FBXW7 through competitively binding to miR-92a-3p in gastric cancer. Mol Cancer 2018; 17: 87.
15.
Zhou Y, Shi H, Du Y, et al. lncRNA DLEU2 modulates cell proliferation and invasion of non-small cell lung cancer by regulating miR-30c-5p/SOX9 axis. Aging (Albany NY) 2019; 11: 7386-401.
16.
Liu J, Yao L, Zhang M, Jiang J, Yang M, Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) 2019; 11: 7830-46.
17.
Mu Y, Tang Q, Feng H, Zhu L, Wang Y. lncRNA KTN1 AS1 promotes glioma cell proliferation and invasion by negatively regulating miR 505 3p. Oncol Rep 2020; 44: 2645-55.
18.
Zhang L, Wang L, Wang Y, et al. LncRNA KTN1-AS1 promotes tumor growth of hepatocellular carcinoma by targeting miR-23c/ERBB2IP axis. Biomed Pharmacother 2019; 109: 1140-7.
19.
Braga EA, Fridman MV, Burdennyy AM, t al. Various LncRNA mechanisms in gene regulation involving miRNAs or RNA-binding proteins in non-small-cell lung cancer: main signaling pathways and networks. Int J Mol Sci 2023; 24: 13617.
20.
Fan Y, Sheng W, Meng Y, Cao Y, Li R. LncRNA PTENP1 inhibits cervical cancer progression by suppressing miR-106b. Artif Cells Nanomed Biotechnol 2020; 48: 393-407.
21.
Jiang Y, Wu K, Cao W, et al. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial-mesenchymal transition by targeting miR-153-3p. Epigenomics 2020; 12: 487-505.
22.
Zhang H, Shao F, Guo W, Gao Y, He J. Knockdown of KLF5 promotes cisplatin-induced cell apoptosis via regulating DNA damage checkpoint proteins in non-small cell lung cancer. Thorac Cancer 2019; 10: 1069-77.
23.
Jia J, Zhang HB, Shi Q, et al. KLF5 downregulation desensitizes castration-resistant prostate cancer cells to docetaxel by increasing BECN1 expression and inducing cell autophagy. Theranostics 2019; 9: 5464-77.
24.
Meng X, Liu K, Xiang Z, Yu X, Wang P, Ma Q. MiR-125b-2-3p associates with prognosis of ccRCC through promoting tumor metastasis via targeting EGR1. Am J Transl Res 2020; 12: 5575-85.
25.
Zhu Y, Ren J, Wu X, et al. lncRNA ENST00000422059 promotes cell proliferation and inhibits cell apoptosis in breast cancer by regulating the miR-145-5p/KLF5 axis. Acta Biochim Biophys Sin (Shanghai) 2023; 55: 1892-901.
26.
Wei R, Zhou Y, Li C, et al. Ketogenesis attenuates KLF5-dependent production of CXCL12 to overcome the immunosuppressive tumor microenvironment in colorectal cancer. Cancer Res 2022; 82: 1575-88.
27.
Li Q, Li S, Li Z, Xu H, Zhang W. KLF5 mediated expression of CARD11 promotes the progression of gastric cancer. Exp Ther Med 2023; 26: 422.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.