Introduction
Osteosarcoma, which is represented by either the direct immature bone genesis or formation of osteoid tissue by tumor cells, is a primary malignant tumor of the skeleton [1]. It is considered as one of the rarest cancers with an incidence of only 4 cases per million annually. Osteosarcoma is more common among children and adolescents [2]. Cancer, in general, is a deviation from a normal cellular program which is associated with abnormalities at the transcriptional level. Therefore, the study of behavior of cancer-related transcripts can help in designing better diagnostic strategies for early detection and prevention of cancer development. Transcriptome studies have revealed that transcription is a surprisingly complex process which results in the production of long non-coding RNA (ncRNA) transcripts which are often overlapping or interspaced between coding and non-coding transcripts [3, 4]. The transcript length of long ncRNA is generally > 200 nucleotides [5]. Long ncRNAs were previously considered as redundant by-products of RNA polymerase II transcription but many studies have revealed that they are involved in a number of regulatory processes including somatic tissue differentiation and tumor development [6–10]. Recently, many studies have focused on the role of long ncRNA in cancer development and metastasis [11, 12]. Our current work was designed to investigate the role of growth-associated long ncRNA 1 (GASL1). GASL1 has already been reported to inhibit the tumor growth in gastric sarcoma by blocking the Wnt/β-catenin signaling pathway [13]. In the present study, the role of GASL1 was explored in osteosarcoma cells along with the underlying mechanism of action. The quantitative reverse transcriptase real-time polymerase chain reaction (qRT-PCR) based expression analysis showed that cancer cells exhibit lower transcript abundance of GASL1. Overexpression of GASL1 long ncRNA resulted in a decline of cell viability and cancer cell migration and invasion. Cell apoptosis was promoted considerably in the GASL1 over-expressing cancer cells. The induction of cellular apoptotic cell death was mediated through modulation of the Bax/Bcl-2 pathway. Western blot analysis showed that the increased expression of GASL1 resulted in a decrease in abundance of phosphorylated PI3K and Akt which resulted in inactivation of the PI3K/Akt signaling pathway and thus inhibition of cancer cell proliferation and metastasis. To sum up, the results of the current study indicate GASL1 to be an important negative regulator of osteosarcoma cell proliferation and metastasis.
Thus, GASL1 downregulation may be used as a biomarker for osteosarcoma and may also prove to be a therapeutic target for the treatment of osteosarcoma.
Material and methods
Culture, maintenance and transfection of cell lines
Osteosarcoma cancer cell lines MG-63, HOS, 143B, T1-7 Saos-2 and the hFOB1.19 human osteoblastic cell line were procured from ATCC (Manassas, VA, USA). The cell lines were maintained as previously reported [14]. A humidified chamber was used for incubation of cell lines at 37°C with 5% CO2. Lipofectamine 2000 was used to transfect the cells either with control pcDNA3.1 or pcDNA-GASL1 construct for 48 h.
RNA isolation, cDNA synthesis and quantitative RT-PCR
The total RNA was extracted using the Pure-Link RNA Mini Kit according to the manufacturer’s protocol. DNA contamination was removed by DNase I treatment. cDNA was synthesized using High-Capacity cDNA Archive Kit. RT-PCR analysis was carried out using the SYBR Green method. The expression of GASL1 was inferred using the GASL1-F (CTGAGGCCA AAGTTTCCAAC) and GASL1-R (CAGCCTGACTTTCCCT CTTCT) primer pair. The amplification conditions were 95°C for 3 min, 40 cycles of 95°C for 15 s, 55°C for 15 s, 68°C for 15 s, followed by 95°C for 1 min, 55°C for 1 min and 70°C for 6 s. The human actin gene was used as an internal control for expression studies.
Assessment of cell viability
The 3-[4, 5-dimethylthiazolyl-2]-2, 5-diphenylte-trazolium bromide (MTT) reagent was used for determination of cancer cells. Briefly, after 0, 12, 24, 48, 96 h cell transfection with pcDNA3.1 and pcDNA-GASL1, the cells were seeded into a 96-well plate at a density of 1 × 106 cells/well and incubated with 0.5% MTT at 37°C for a period of 4 h. To solubilise MTT-formazan, 150 µl of DMSO was added to each well. The absorbance at 570 nm was recorded.
Colony formation assay
The cells were grown in 6-well plates for 12 h until the density reached 1 × 103/100 µl of medium. This was followed by 48 h incubation at 37°C with 5% CO2 concentration. The cell pellet was harvested by centrifugation and washed with PBS buffer. Each cell pellet was resuspended in 1 ml of 0.1% crystal violet solution and incubated for 30 min. The cells were again collected by centrifugation and washed with PBS buffer until the suspension became clear. Then, the colonies were counted and pictures were taken.
AO/EB staining for assessment of cell death
The acridine orange/ethidium bromide (AO/EB) staining method was used to estimate the apoptotic cell death. In brief, Saos-2 and MG-63 cells transfected with pcDNA3.1 and pcDNA-GASL1 were plated in 96-well plates at a density of 2 × 105 cells/well. An aliquot of 25 µl from each well was mixed with 1 µl of dye phosphate buffered saline solution containing 100 µg of each acridine orange and ethidium bromide (both supplied by Sigma Chemical Company). About 10 µl of this mix was put on a glass slide, covered with a cover slip, and nuclear morphology was visualized under a fluorescent microscope.
Analysis of cell migration
The migration ability of cancer cells was assessed by the wound-healing method, as previously described by Ma et al., 2018 [15]. Briefly, the normal control (NC) and transfected cancer cells were cultured in 6-well plates. A perpendicular scratch was made on the cell surface using a 200 µl pipette tip. The scratch area was photographed at this stage and also after 24 h incubation at 37°C in a 9% CO2 environment.
Cell invasion assay
Transwell was used to analyze the invasiveness of NC and transfected osteosarcoma cells. Cancer cells were cultured in transwell chambers. The latter were put into 24-well plates containing culture medium having 20% FBS and incubated for 2 days at 37°C. Cotton swabs were used to remove any non-invading cells from the upper surface of the insert. Cells which invaded were fixed using methanol and stained with 0.5% crystal violet for 35 min. The counting of invading cells was performed for at least 10 random field views under a high magnification light microscope.
Western blotting
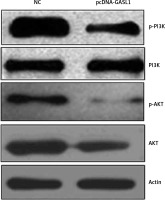
Cancer cells were transfected with pcDNA3.1 and pcDNA-GASL1 and cultured for 24 h. Centrifugation was done to collect the cell pellet which was lysed using RIPA buffer. Bradford reagent was used to determine the protein concentration. About 30 µg of protein from each lysate was loaded on SDS-PAGE. The PAGE gel was blotted to PVDF membrane and final detection was performed using primary Akt (cat. no.sc-135829), phosphorylated (p)-AKT (cat. no. sc-7985-R), PI3K (cat. no.sc-136298) (all 1 : 1,000; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and secondary antibodies (horseradish peroxidase-conjugated anti-rabbit secondary antibody (sc-2357-CM)).
Statistical analysis
For each experiment, at least three independent replicates were performed. The final values are presented as mean ± standard deviation. Student’s t-test was performed using GraphPad Prism 7 software to compare the values. P < 0.05 was indicative of a statistically significant difference.
Results
GASL1 is downregulated in osteosarcoma
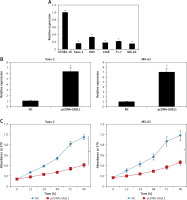
Expression analysis of lncRNA GASL1 by qRT-PCR in normal osteoblast cells and osteosarcoma cell lines showed that GASL1 exhibits significantly lower expression in cancer cells (Figure 1 A). This reveals that osteosarcoma proceeds with lowering of GASL1 expression.
Figure 1
A – Expression of lncRNA GASL1 in normal hFOB1.19 and different osteosarcoma cell lines. B – Expression of GASL1 in NC and pcDNA-GASL1 transfected Saos-2 and MG-63 cells. C – Cell viability of NC and pcDNA-GASL1 transfected Saos-2 and MG-63 cells. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)
Overexpression of GASL1 inhibits osteosarcoma cell proliferation
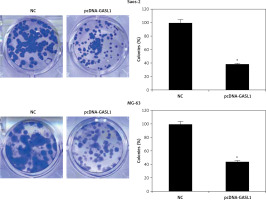
The overexpression of GASL1 in Saos-2 and MG-63 cancer cell lines was achieved by using the pcDNA3.1 vector via the transfection method. The stable transformation of GASL1 was confirmed by nearly 6-7 fold enhanced expression of the former in cancer cells in comparison to pcDNA3.1 vector transfected (NC) cells (Figure 1 B). To assess the viability of NC and GASL1 overexpressing cancer cells, cells were transfected and processed for MTT assay at 0, 12, 24, 48 and 96 h of transfection. The absorbance was taken at 570 nm, which is indicative of viability of cells, and it was seen that NC cells had higher O.D. in comparison to GASL1 overexpressing Saos-2 and MG-63 cells and the difference was more notable at longer durations of transfection (Figure 1 C). This suggests that cancer cell viability decreases with increase in GASL1 expression. This was also confirmed by remarkably low clone forming ability of GASL1 overexpressing cancer cells (Figure 2).
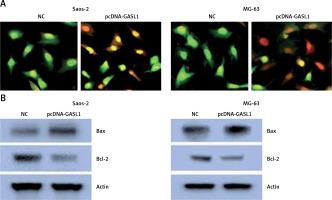
Elevation of GASL1 expression leads to Bax/Bcl-2 mediated cell apoptosis
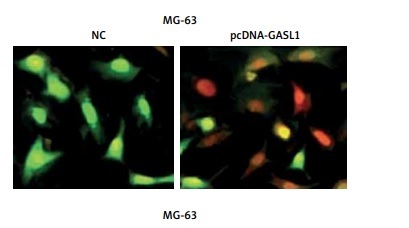
The acridine orange/ethidium bromide (AO/EB) staining method was employed for analysis of cell apoptosis in cancer cells. The fluorescence microscopic investigation of NC and Saos-2 and MG-63 cell lines overexpressing GASL1 showed deformation of chromatin material (Figure 3 A). To check whether overexpression of GASL1 in cancer cells modulated the Bax/Bcl-2 apoptotic signal, western blotting was performed. The expression of Bax protein was elevated while Bcl-2 expression was downregulated (Figure 3 B).
Figure 3
A – AO/EB staining showing the induction of apoptosis in Saos-2 and MG-63 cells transfected with NC or pcDNA-GASL1. B – Western blots showing the expression of Bax and Bcl-2 in NC and pcDNA-GASL1 transfected Saos-2 and MG-63 cells. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)
GASL1 overexpression inhibits migration and invasion of osteosarcoma cells
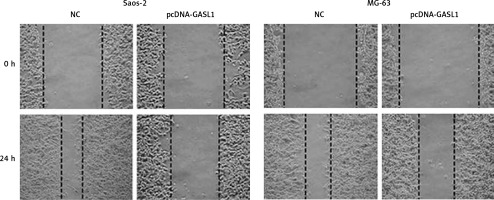
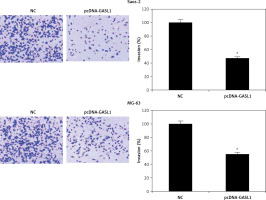
Cell migration and invasion are crucial to metastasis. Wound healing and transwell experiments were performed for assessment of migration and invasion of osteosarcoma cells. The results showed that cell migration was inhibited dramatically in GASL1 overexpressing cancer cells (Figure 4). GASL1 overexpression also resulted in a decline in the invasion of osteosarcoma cells. The percentage of invasion decreased to only 45% and 57% in GASL1 overexpressing Saos-2 and MG-63 cells, respectively, in comparison to respective NC cells (Figure 5).
Overexpression of GASL1 inactivates PI3K/Akt signaling pathway
Finally, to analyze the underlying molecular mechanism of inhibition of cancer proliferation under GASL1 overexpression, western blotting of PI3K, phosphorylated PI3K (p-PI3K), Akt and phosphorylated Akt (p-Akt) was performed. The protein expression showed that GASL1 overexpression hardly had any effect on PI3K and Akt protein levels but there was a surprising decrease in p-PI3K and p-Akt protein concentrations (Figure 6). This is suggestive of inactivation of PI3K/Akt signaling upon overexpression of GASL1 in osteosarcoma cells. The effects of PI3K/Akt signal modulation are evident as reduction of cancer cell proliferation and metastasis.
Discussion
Osteosarcoma is one of the rarest malignant tumors. Of the all biopsy-analyzed malignant bone tumors, it has a frequency of only about 15% [1]. A number of studies have proven long ncRNA as an important regulators of cancer development but there is hardly any such report for osteosarcoma. GASL1 ncRNA has already been shown to inhibit cell proliferation by inducing cell apoptosis. The downregulation of GASL1 resulted in cell cycle progression via the promotion of G1 exit in cancer cells [16]. It also inhibited the progression of lung cancer by blocking the TGF-β signaling pathway [17]. A recent study showed that GASL1 has an inhibitory role in prostrate carcinoma [18]. In order to unravel the role of GASL1 in osteosarcoma, expression analysis was carried out in infected bone tissue and non-infected surrounding tissue and it was observed that GASL1 has significantly lower expression in the infected tissue, suggesting its probable regulatory role in osteosarcoma. This was also evident from higher expression of GASL1 in human osteoblast cells in comparison to osteosarcoma cancer cell lines. Analysis of cell viability showed that overexpression of GASL1 resulted in loss of cell proliferation and promoted the apoptosis of cancer cells. The reduction in cell proliferation of cancer cells was mediated via blockage of the PI3K/Akt signaling pathway. The western blotting analysis showed that GASL1 exerts its negative regulatory effect by inhibiting the phosphorylation of PI3K and Akt. This in turn inhibits the expression of downstream genes important for cell proliferation and cancer metastasis. When GASL1 was overexpressed in osteosarcoma cancer cells, their ability to migrate and invade was reduced to a considerable level. This indicates that metastasis of osteosarcoma progresses with reduction in the transcript generation of GASL1 and by increasing the expression of GASL1, progression of osteosarcoma is halted. Further, inactivation of PI3K/Akt pathway induced cell apoptosis has already been established by Chao et al. in 2010 [19] and our results also suggest the same. GASL1 induced apoptosis is also clear from protein expression of Bax and Bcl-2 apoptosis related protein. Bax was seen to be overexpressed while Bcl-2 protein concentration was reduced considerably. Taken together, the results indicate that GASL1 acts as a negative regulator in osteosarcoma cancer progression. Its anticancer role is evident as its ability to inhibit the proliferation and metastasis of osteosarcoma cells via modulation of the PI3K/Akt signaling pathway and to promote cell apoptosis by modulating the Bax/Bcl-2 ratio.
In conclusion, osteosarcoma at the molecular level proceeds with downregulation of long ncRNA GASL1. The overexpression GASL1 in cancer cells inhibited cell proliferation, migration and invasion by inactivating PI3K/Akt signaling. The study may pave the way for utilization of LncRNA GASL1 for the treatment of osteosarcoma.