Introduction
Early studies have indicated that approximately half of patients receive the recommended care in chronic conditions, and this remains true when considering screening, diagnosis, treatment and follow-up [1]. Data regarding guideline adherence are important because they provide comparative benchmarks and identify potential areas of intervention [2]. In concordance with “treat-to-target” principles [3], evidence-based European League Against Rheumatism (EULAR) guidelines for rheumatoid arthritis (RA) advocate early and individualized treatment [4]. In comparison with routine care, tight control through more objective and frequent patient assessment was shown to improve clinical outcomes at little cost [5]. Rheumatologists are the assessors and the main decision makers when undertaking “tight control” to achieve remission, and their compliance with guidelines is of paramount importance. Sub-analyses of trials in RA have shown that physician adherence to tight control additively influences remission rates and working capacity [6]. In another recent trial, T2T adherence led to reduced disease activity and higher remission rates, while physician adherence was the only predictor of remission at 2-year follow-up [7]. Real-world studies have reported a considerable “evidence-practice” gap, with the majority of patients managed with inadequate disease control [8]. Research suggests that despite prior education and awareness of the “treat-to-target” (T2T) strategy, rheumatologists may still not fully comply in clinical practice [9, 10]. It has been suggested that time constraints, a shortage of specialists and budget limitations impede the application of T2T in real-world practice [11]. Potential barriers also include a lack of confidence in composite measures [12], and even poor patient-provider communication [13]. All these studies agree in that rheumatologists support T2T overall, but may not fully comply with particular recommendations, which is in line with multinational surveys [14]. In an Italian cross-sectional study, adherence to recommendations regarding methotrexate safety was good, while dosage and time of treatment initiation were suboptimal [15]. There are limited data regarding the practical challenges faced by rheumatologists in a real-world setting. The existing data are not easily comparable between countries due to health care system differences (financial and organizational), while Polish data are lacking. Potential interventions have to be tailored to national areas of unmet need, to the extent by which they are valued from a payer’s perspective. We previously characterized the epidemiology of RA in the first population-based study, which revealed an increased burden exceeding earlier projections, suboptimal control of disease and limited access to biologics [16]. This research was inductive, designed to describe the daily routine of Polish rheumatologists in the management of biologic disease-modifying anti-rheumatic drug (bDMARD)-naïve patients with the RA diagnosis established at least 12 months earlier. Considering that only 3% of the Polish RA population have access to bDMARDs [16], this population represents the vast majority of the RA population.
Material and methods
A representative sample of 101 Polish rheumatologists was recruited. Rheumatologists were invited in person by interviewers to answer an online questionnaire using computer-assisted web interviewing (CAWI).
Eligibility criteria for rheumatologist inclusion in the research were defined as follows:
– provision of care to at least 25 adult bDMARD-naïve RA patients, diagnosed at least 12 months earlier;
– initiation of non-biologic RA treatment in patients whom they managed from first-admission therapy.
Random quota sampling was used to select rheumatology outpatient care centers from a national database. All centers having a National Health Fund (NHF) contract for RA treatment, regardless of whether they provided biologic treatment or not, were included. The sample was allocated across different layers. These were defined by geographical distribution (division into voivodeships and city type), and the size of the NHF contract (3 groups of centers equal in size), and corresponded to the distribution of rheumatology centers. Based on these layers, 100 centers were randomly selected (the target sample size), and the starting points, the first places the interviewers went to invite rheumatologists to participate, were chosen. For each randomly selected starting point, a list of substitute centers was arranged in its geographical proximity. Geographical proximity was determined based on postal codes.
After all selected centers and substitute centers were included, sampling was carried out by quota, irrespective of established layers. This type of selection was necessary for 30% of the sample due to lack of consent. All data collected during the research were declarative, including prevalence estimates. Twenty-eight specialists were otherwise employed in centers offering reimbursement programs for bDMARDs. No data from the patients’ medical records were collected and no additional diagnostic or therapeutic interventions were involved. For the purpose of the research, rheumatologists were provided with a paper survey and an electronic questionnaire, available online. Interviews were completed from 16 November 2017 to 20 December 2017.
The questionnaire consisted of five parts. The first included 12 questions describing the clinical profile of patients. Data gathered involved e.g., the frequency of visits to the rheumatologist, the impact of the disease on the professional activity of the patients, and the criteria used for assessing disease activity. The second part of the questionnaire focused on general issues associated with RA treatment, and contained 34 questions. The third part, including 28 questions, was dedicated to the analysis of RA therapy-related general practice with particular medications or treatment regimens. The fourth part dealt with reasons for withdrawal of selected medications, and contained 12 questions. The last part of the questionnaire assessed rheumatologists opinions on the usage of glucocorticoids in addition to non-biologic RA treatment in 5 questions.
Results
The survey results describe rheumatologist statements regarding management of bDMARD-naïve patients with an RA diagnosis established at least 12 months earlier. Differences between clinical practice and EULAR recommendations are summarized in Table I.
Table I
Nonconformity with EULAR recommendations in clinical practice in Poland
Disease activity assessment
Rheumatologists estimated disease activity in patients with first-admission treatment to be high in 43%, moderate in 41% and low in 16%. Disease activity was assessed every 2–3 months by 62% of rheumatologists and monthly by 5% of specialists. Thirty percent of rheumatologists measure disease activity every 4–6 months.
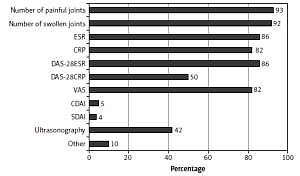
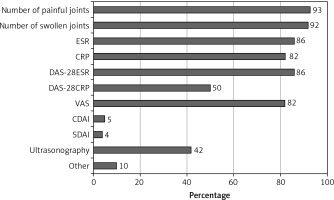
The RA activity was most often assessed using the swollen and tender joint count (SJC/TJC) (92–93%) and physical examination (87%). Of the composite indices, the 28-joint disease activity score based on erythrocyte sedimentation rate (DAS28-ESR) was more commonly applied than the score based on C-reactive protein (CRP) (86% and 50%, respectively). In addition, 4–5% of rheumatologists used the simplified and clinical disease activity indices (SDAI, CDAI). Measures of disease activity are described in detail in Figure 1.
When determining remission, rheumatologists considered the following as the strongest indicators: SJC/TJC (46%), normalized ESR and CRP (43%), and DAS28 < 2.6 (38%). Of the rheumatologists who confirmed the use of ESR, 67% stated that it does not determine disease activity by itself. The same claim was made by 60% of rheumatologists for both CRP and Patient Global Assessment (PGA). Forty-two percent of rheumatologists use ultrasound (US) when assessing disease activity.
Conventional therapy in bDMARD-naïve patients
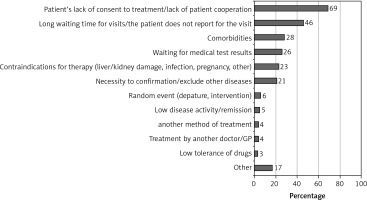
No treatment was initiated at the time of diagnosis in 15% of patients, for 29% of whom treatment commencement was delayed for over 3 months. Lack of patient consent/cooperation (69%) and long waiting queues for rheumatologist appointments (46%) were the most frequently reported reasons (described in detail in Figure 2).
Sixty-four percent of bDMARD-naïve patients have never achieved remission. The time to first-ever remission was over 6 months from treatment initiation for 54% of patients, and only 17% achieved remission in less than 3 months. Among patients who experienced 2 or more switches of treatment schedules, 30% did not achieve remission. Of the latter, 34% did not have treatment with biological agents planned. Most difficulties in enrolling patients for biologic therapy are due to non-medical factors, and among them contraindications to bDMARDs (39%) and reimbursement barriers (26%) are the most common (for further details see Figure 3).
Figure 3
Impediments to qualifying patients for biologic therapy despite active disease following 2 therapy modifications (n = 74)

The classical synthetic DMARDs (csDMARDs) most frequently prescribed by rheumatologists, regardless of time from diagnosis, were methotrexate (MTX) (100%), sulfasalazine (92%), and leflunomide (89%). Rheumatologists judged MTX to have the highest efficacy in achieving remission. Among csDMARDs, the proportion of patients who did not achieve remission was 34% for MTX (lowest for all drugs, data not shown), 43% for leflunomide, and 59% for sulfasalazine. An MTX dosage of 25 mg/week was the most frequent (73%) highest dose in monotherapy. At this point, rheumatologists consider it an insufficient response and change therapy.
The MTX was included in 98% of first-treatment options: 81% commence with oral MTX and 17% with subcutaneous MTX. The first-line strategies most frequently applied were: oral MTX monotherapy (35%), oral MTX + glucocorticoids (GC) (19%), and oral MTX + GC + nonsteroidal anti-inflammatory drugs (19%). Rheumatologists estimated the proportion of patients to achieve remission at 56%, 66% and 61% respectively. MTX was excluded as a first-line therapy most often due to contraindications (49%) and low disease activity (21%). Second-line treatments most commonly comprised leflunomide, subcutaneous MTX, and sulfasalazine. Most rheumatologists estimated that the mean time of inadequate response before they switch therapy is 3–6 months (49%), while 19% stated 6–12 months.
Seventy percent of rheumatologists declared that the therapeutic use of GC lasts > 3 months and > 6 months for every fourth patient. Thirty percent of responders use GC therapy for 1–3 months.
The most common reasons reported for GC use duration > 3 months were lack of remission and disease activity increase despite DMARD treatment (40% and 38%, respectively). An estimated 72% of patients taking GC receive doses ≤ 7.5 mg/day, and 42% receive ≤ 5 mg/day.
The most common sources of reference for rheumatologists regarding RA management were the EULAR and ACR guidelines, personal experience, and scientific publications, in that order.
Clinical and productivity-oriented outcomes
Rheumatologists estimated that, at present, the majority (44%) of their bDMARD-naïve patients are characterized by inadequate disease control, with only 21% in sustained remission.
The general symptoms most often reported by patients include fatigue (55%), mood disturbances (33%), and anemia (32%). Long-lasting concomitant treatment for other comorbidities was estimated to be prescribed in almost 60% of patients, including osteoporosis treatment and cardiovascular disease prophylaxis (68% and 36%, respectively).
Sixty-five percent of rheumatologists estimate that the mean annual sick leave is 6–30 days, and 29% considered that RA does not limit their patients’ work ability.
Discussion
The present research provides a unique and wide scope of insights into the management patterns of RA, in which it may serve as a benchmark and provide a basis for tailored interventions. To our knowledge, we have provided the first representative Polish data on routine care of RA from a provider’s viewpoint. We describe several areas of practice where nonadherence to guidelines may occur. There remains a considerable proportion of patients with inadequately controlled RA, in whom management may not be optimal, while a variety of medical and nonmedical challenges still exist.
Initiation of DMARD therapy once a diagnosis is established and the achievement of remission within the first 6 months are the cornerstones of RA management [4, 17, 18]. Indeed, achieving clinical remission by established indices has been demonstrated to improve radiographic and clinical outcomes in early RA [19]. Rheumatologists participating in a multinational survey were reported to prescribe a DMARD within 4 weeks of diagnosis in 67% of patients [9]. A pooled analysis of international data showed that the median lag time from diagnosis to DMARD initiation was 2.14 months (range: 0–2.2) [20]. In the context of our current findings, rheumatologists estimated that, on average, 85% of patients are assigned treatment at the time of diagnosis while of the remaining, 1/3 have a delay of over 3 months. Previous studies published in 2017 demonstrated that only 30% of patients in Poland are diagnosed within 3 months of symptom onset, while the average delay for the country was estimated to be 9 months [16]. When considering the lag time from symptom onset to diagnosis, and then on to treatment, there remains a significant proportion of Polish patients excluded from the therapeutic “window of opportunity”. Priority in further research should be given to establishing a nationwide strategy for patients to receive a diagnosis and treatment within this desirable interval, where most RA patients might benefit.
Patient and provider communication is often underappreciated, despite shared decision-making emphasized as an overarching EULAR principle, and meta-analyses demonstrating that poor communication decreases adherence rates [21]. In addition to long waiting queues, 69% of rheumatologists emphasize difficulty in obtaining patient consent or cooperation. Nevertheless, higher awareness of this issue should translate into an education framework for rheumatologists. Educational interventions have been demonstrated to reduce physician non-adherence [22]. In a review by Wabe et al., medication side effects, comorbidities and persisting disease were among the factors associated with physician nonadherence to T2T [23]. We provide evidence that comorbidities and contraindications to RA-dedicated drugs are among the most prevalent reasons for delayed DMARD treatment. An example is the use of MTX in hematologic or hepatic abnormalities, which may be present at disease onset. It should be recognized that the waiting time for laboratory and imaging test results can also account for the delay.
Rheumatologists estimated that at present only 21% of bDMARD-naïve patients have adequate disease control (DAS28 ≤ 2.6). Similarly, previous cross-sectional data demonstrated that 26.1% of Polish patients are in remission, including 3% of all patients with RA treated with bDMARDs [16]. This suggests that rheumatologists are aware, at least in their own practice, of the scale of inadequate disease control in RA patients. Unfortunately, if remission less than 6 months from diagnosis is attained only by 46% of patients, it means we are failing in “treating to target”. Notably, 30% of patients treated with at least 2 different courses of therapy were estimated not to achieve remission and 34% of them do not have bDMARDs planned for future treatment. The latter finding is particularly worrying when considering that all responders stated that they rely on EULAR guidelines when making treatment decisions. Studies previously showed that declarative awareness does not determine clinical application, even with previous training [9]. Although we cannot exclude personal convictions, rheumatologists identified several non-medical difficulties in initiating bDMARD treatment: 26% stated reimbursement barriers, 15% described patient fear of bDMARDs, and 4% of specialists themselves had concerns about bDMARD safety profiles. The identification of these issues in the present research may prompt policy makers to institute systemic changes and rheumatologists to more thoroughly address patient doubts. Vermeer et al. concluded that T2T is a feasible strategy for daily clinical practice based on a cohort of RA patients [24]. Overall T2T adherence was observed in close to 70% of visits; however, discordance was observed when rheumatologists did not agree with disease assessment indices. In 2011, an international survey revealed that rheumatologists agreed least with the frequency and composite index use when monitoring in accordance with guidelines [14]. In a survey among Canadian rheumatologists, some responders stated that tight control was unnecessary [12]. At present, although 62% of rheumatologists stated that they monitor patients every 2–3 months, the majority are reported to never achieve remission, which indicates inadequate management. Strikingly, only 5% of responders specified that they monitor disease activity monthly, despite 41% of patients reportedly having highly active RA at diagnosis. This may suggest a lack of compliance with the principle of “tight control” and monitoring every 1 to 3 months until remission is reached. In a EULAR congress survey [25], 68% of responders stated that treatment success and remission were measured through improvement in composite indices, and acute-phase reactants were among the most frequently evaluated disease measures. One-third of participants relied on their own judgment when assessing RA activity. In other countries, composite index use received less support than in international surveys, and was considered by some to be too complicated for daily practice [12]. In the United Kingdom, CRP assay was performed monthly in 60% of patients, while DAS-28 was assessed only in 25% [26]. In a Japanese survey, rheumatologists were found to have a tendency to interpret individual rather than composite measures, while reasons for not calculating DAS28 included time shortage and lack of staff [13]. For overall disease activity assessment, Polish rheumatologists seem to place the highest value in joint evaluation (TJC/SJC), with acute-phase markers and DAS28-ESR also frequently applied. However, when determining remission, reliance on DAS28 < 2.6 as the strongest indicator was reported only by 38% of specialists. This is concerning because personal judgment may be less sensitive to subclinical disease activity, and composite indices may more precisely determine progression. Furthermore, if only 60–67% of specialists consider acute phase molecules as singularly unreliable determinants of disease activity, the remaining specialists may inadequately monitor and adjust therapy using ESR and/or CRP. The CDAI use by only 5% of rheumatologist is striking, especially since it was developed for simplicity in daily practice. However, similar challenges are prevalent internationally. Only 27% of Japanese rheumatologists use validated composite indices of remission as a target of treatment [13]. In the United Kingdom, outside of early arthritis centers, monthly CRP is assessed in 70% of patients while DAS28 is assessed only in 13% [26].
Of note is the relatively high proportion (42%) of responders using US as a disease activity measure in RA. This may indicate the rising number of rheumatologists skilled in performing US or having access to an office-based US machine. To an extent, US assessment may also bypass the need for laboratory testing, which may be more time-consuming.
Remarkably, 98% of reported first-line treatment schemes included MTX (81% oral, 17% subcutaneous), which is the currently recognized anchor drug. Although preference in the initiation form of MTX is not indicated in current guidelines, randomized trials report a potentially higher efficacy of the subcutaneous route [27]. In practice, rheumatologists seem to treat subcutaneous MTX monotherapy as the first modification to therapy (12%), though leflunomide use (17%) is more common. The most common first therapy modifications included leflunomide monotherapy, subcutaneous MTX and sulfasalazine. In both mono- and polytherapy, the target dose of 25 mg of MTX per week was estimated to be reached by most patients. When considering treatment schemes, rheumatologists seem to practice in accordance with EULAR guidelines. However, an area of inconsistency is long-term GC use. The GC schemes lasting over 6 months are reportedly applied in 25% of patients. However, GCs are recommended only for short-term use and with rapid dose reduction [4].
High disease burden is recognized to affect patient quality of life and productivity loss [28, 29]. We conclude that rheumatologists recognize the prevalence of systemic RA-related symptoms and comorbidity burden. Most specialists (65%) estimated that the mean annual time spent on sick leave is between 6 days and 1 month. In comparison, social insurance data show that RA patients require 2 episodes of sick leave on average, with a mean duration of 17 days, indicating that actual time spent on sick leave is closer to 1 month [29]. Rheumatologists may have underestimated this time period, since patients can request sick leave from their GPs and other specialists. To illustrate the importance of these estimates, seropositive RA was shown to be the most costly autoimmune disease to cause sick leave, which in itself contributes to about 40% of total RA costs, alongside disability [29].
The major limitation of the research is the survey design, which is based on individual claims. The findings were not compared with medical records; therefore they are based on the credibility of responses and associated bias. The wide scope of the investigation did not allow each aspect of management to be assessed critically, and can only provide insights from a physician’s viewpoint. Conclusions drawn should be treated cautiously, as follows from the introductory character of this study. Estimates provided may be inconsistent with medical records [9]. Assessing adherence of physicians is difficult, and is directly incomparable between studies due to a lack of standardization in assessments [23]. The questionnaire we used was not designed to assess adherence alone, but was developed in the perspective of RA management according to guidelines. The population is likely representative of Polish rheumatologists due to the random quota sampling selection of outpatient clinics and then of rheumatologists. However, quota sampling was necessary for 30% of the research sample during recruitment. Notably, 28% of specialists were otherwise employed in centers with access to biologic treatment, and, therefore, potentially had experience with recruitment to bDMARD reimbursement programs.
In conclusion, nonconformity with evidence-based recommendations is centered on frequency and applied measure of disease activity monitoring, time to DMARD initiation, and duration of GC treatment. Difficulties in the optimal management of patients stem from a variety of non-medical factors.