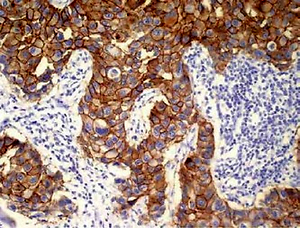
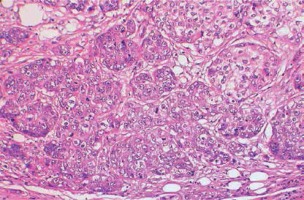
Introduction
According to the prevalent epidemiological data, breast cancer (BC) is the most frequent female cancer worldwide. The multitude of clinical and pathological cases considered in creation of the World Health Organization (WHO) classification of breast neoplasms shows the complexity of the medullary breast cancer (MdBC) issue. MdBC is one of the most uncommon BC subtypes, representing 1–7% of all cases [1]. Its absolute prevalence, reaching every year around 4,900 cases worldwide, makes it a considerable proportion of the general cancer cases. Recent findings show MdBC to be a heterogeneous, spectrum-based group of various lesions rather than one strictly classified pathological diagnosis, and these neoplasms (typical medullary, atypical medullary and medullary-like invasive ductal carcinoma) are classified together as “carcinoma with medullary features” or simply “medullary breast cancer” [2]. Many studies have confirmed MdBC to exhibit a triple-negative phenotype, lacking progesterone (PR), estrogen (ER) and HER2 receptor expression. Usually triple-negative phenotype is associated with an aggressive clinical course, and is considered to be more resistant to chemotherapy and more likely to metastasize than other molecular breast cancer types. Moreover, it is associated with a lower disease-free survival rate and shorter life expectancy [3]. METABRIC research, considered to be the largest global study on breast cancer molecular issues, showed a significantly elevated rate of TP53 mutations in comparison to other triple-negative cancers as the only significantly feature shared by the whole range of MdBC tumors [4]. MdBCs are in general associated with histopathological features widely acknowledged as “aggressive” – high mitotic index, enriched cytoplasm, easy syncytia formation and a very high level of genome instability. What is more, MdBCs are usually made of poorly differentiated cells characterized by the presence of large nuclei and prominent nucleoli. Surprisingly, patients with MdBC present significantly longer 5- and 10-year survival than patients suffering from other BC types. Higher frequency of rearranged genes is believed to be responsible for such an outcome. While inconsiderable genetic shifts are essential for cancer cells to be promoted and to avoid an immune system response, greater accumulation of mutations allows tumor epitopes to differ from unaffected ones relevantly enough to be recognized, infiltrated and confined by immune cells. This is the reason why MdBC should not be evaluated in Scar-Bloom-Richardson system, and if it is, the clinical outcome hardly corresponds with the most commonly assessed high grade [1].
Typical medullary breast carcinoma, whose cells present all of the aforementioned MdBC features, occurs more commonly among patients with BRCA1 mutation, but only about 13% of women with MdBC have this mutation. Recent studies suggest that the phenomenon of gene methylation might be responsible for flawed BRCA1 expression [4, 5].
The aim of this study was to revise the histological and pathological features of MdBC and to analyze ER, PR and HER2 expression in order to make a comparison to invasive ductal breast cancer (IDC), which comprises the vast majority of diagnosed BCs.
Material and methods
The material for the study was composed of histological preparations obtained from 1,122 females diagnosed and treated for invasive breast cancer. MdBC was identified in 12 out of 1,122 women diagnosed with invasive BC in our center between 2009 and 2011. Clinical and demographic characteristics of patients were retrieved from the patient files. The biological material for the study derived from excisional breast biopsies and radical mastectomies. Tumor samples were fixed in 10% phosphate buffered formalin. After 24 h, fixated samples were dehydrated in alcohols of gradually increasing concentrations (50, 60, 70, 80, 90, and 96%), followed by pure alcohol and xylene. Afterwards, tissues were embedded in paraffin. Paraffin blocks were cut into sections, with a thickness of 4 µm each. The acquired samples were stained with different histopathological methods. Preparations stained with hematoxylin and eosin were used to identify histological type of cancer (WHO classification), histological grade of malignancy, and intensity of divisions expressed as the mitotic index of cancerous cells (the average number of mitoses in cancerous cells counted in 10 fields of vision at 400× objective magnification (surface field 0.17 mm2)) [6].
Routinely, patients had a basic molecular profile evaluated, i.e. ER, PR and HER2 expression. Immunohistochemical procedures recruited paraffin samples placed on glass slides covered with 2% silane (Merck, Darmstadt, Germany) and dried for 24 h at 42°C. Prior to initiating the immunohistochemical procedures, samples were dewaxed by placing them in a series of alcohols of gradually decreasing concentrations (96, 90, 80, 70, 60, and 50%), and subsequently washed in distilled water. Immunohistochemical assays were performed using the En-Vision complex HRP Cytomatic (DAKO, Santa Clara, United States) (En-Vision Dual Link System-HRP, DAB, Code: K4065).
In order to define the expression of receptors for steroid hormones (ER, PR), monoclonal antibodies against estrogen receptor (Monoclonal Mouse Anti-Human Estrogen Receptor alpha, 1 : 50 dilution, Clone: 1D5, Code: IR654, DAKO, Santa Clara, United States) and progesterone receptor (Monoclonal Mouse Anti-Human Progesterone Receptor, 1 : 400 dilution, Clone: PgR636, Code: IR068, DAKO, Santa Clara, United States) were used [6]. Samples were incubated at 60°C and then dewaxed. Afterwards, the cancerous epitopes were revealed by warming samples in a buffer for 40 min. Next, preparations were left at room temperature for 20 min, followed by rinsing them in a buffer, and endogenous peroxidase was blocked in 3% hydrogen peroxide. Subsequently, preparations were incubated with a dedicated antibody. Afterwards, samples were rinsed in a buffer for 10 min and then incubated with the reagent (Visualization Reagent) for 0.5 h. After that, preparations were washed in TBS (Tris-Buffered Saline, Code: S1968), pH 7.6, for 10 min, and then incubated with 3,3’-diaminobenzidine (DAB) (Substrate-Chromogen Solution) for a further 10-minute period to evoke the color reaction. Finally, hematoxylin preparations were stained and preparations were immersed in Canadian balm. Afterwards, the color reactions were assessed in accordance with the scale that takes into account the extent and intensity of staining of cancer cells’ nuclei. Nuclear staining in > 10% of cells was regarded as positive (+) for ER and PR [6].
HER2 expression was defined by using the Hercept Test (Code: K5204, Dako, Santa Clara, United States) utilizing a polyclonal antibody against HER2 (Rb A – Hu HER2 – Rabbit Anti-Human HER2 Protein). HER2 state was defined by evaluating its expression on the cancerous cell membranes using immunohistochemistry, and in some cases (2+) proved by estimating the number of HER2 gene copies employing fluorescence in situ hybridization (FISH). The HER2 expression rate was established based on the maximum surface staining intensity, as follows: prominent peripheral membranous staining > 30% of tumor cells was designated 3+; temperate peripheral membranous staining in ≥ 10% of cancer cells or prominent perimetric membranous staining in ≤ 30% of cells was graded as 2+; poor and incomplete membranous staining was marked as 1+; and no staining was scored 0. Scores of 0 and 1+ were both regarded as negative for HER2 amplification. A score of 3+ was considered as positive. A score of 2+ was considered equivocal and FISH was applied for confirmation. HER2 was considered to be amplified if the median HER2 copy number was ≥ 6 signals/cell or the ER2/CEP17 ratio was ≥ 2 [7]. Positive and negative control preparations were determined beforehand using techniques mentioned above.
Statistical analysis
Statistical analyses were performed using the Statistica 13.1 package. Biographical information was condensed using descriptive statistics (mean, median, range and standard deviation). The chi-square (χ2) test with Yates’ correction or Fisher’s exact test, when the predicted cell counts were < 5, were used to compare categorical variables. The obtained results were considered statistically significant if p ≤ 0.05.
Results
We analyzed 12 MdBCs representing 1.07% of a total of 1,122 females suffering from invasive BC qualified for the study. The mean age of the studied group of patients was 58.54 years (range: 30–70 years). Patients were divided into 7 age groups: ≤ 30; 31–40; 41–50; 51–60; 61–70; 71–80; and ≥ 81 years (Figure 1). Among all 1,122 investigated invasive BCs, a wide range of histological subtypes was found. IDC comprised the largest subgroup (76.29% of cancers) (Figure 2), followed by lobular (14.08%) and mixed ductal and lobular cancers (3.65%). Metaplastic, mucinous, tubular, medullary and micropapillary cancers were much less frequent (Table I). Clinicopathological findings of all MdBC cases are summarized in Table II. The average diameter of the primary MdBC foci was 2.05 cm (range: 1.2––3.5 cm). The MdBC group demonstrated a larger median tumor diameter than the IDC group (2.05 cm vs. 1.89 cm), although ≥ T2 tumors comprised 42% vs. 51%, respectively. The right breast was involved in 5 patients, the left one in 7. All analyzed MdBCs were monofocal. Most patients (58%) had T1c disease (which means tumor > 10 mm but ≤ 20 mm in the greatest dimension). In the study we also assessed the regional lymph nodes status, finding that in both examined BC groups (MdBC, IDC) women without regional lymph node involvement (pN0) (83%; 57%, respectively) comprised the largest group. Postoperative microscopic examination proved regional lymph nodes metastasis only in 2 (17%) MdBC cases. The present study shows a statistically significant difference in the presence of nodal involvement between MdBC and IDC groups (p < 0.001).
Table I
Distribution of histological types in the group of 1,122 patients with invasive breast cancer
Table II
Clinicopathological features of 12 medullary breast cancer (MdBC) cases
Figure 1
Age distribution in the medullary breast cancer (MdBC) group and the invasive ductal breast cancer (IDC) group of patients
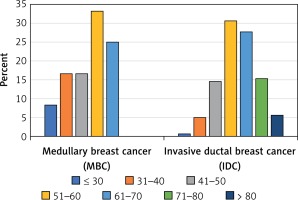
Figure 2
Histopathological image of invasive ductal breast carcinoma (IDC) with central necrosis, grade 3 (400× magnification)
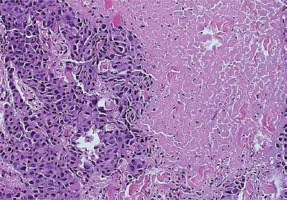
Depending on the histological grade of malignancy, MdBC showed a statistically higher grade of histological malignancy (G1–G3) (p = 0.003) compared to IDC (Figure 3). Most MdBC cases were grade II tumors (G2) (93%), similarly to the IDC cluster, in which G2 tumors accounted for 59%. A significant difference can also be found when analyzing highly differentiated tumors. 9% of IDCs were assessed as G1 tumors, while there were non-differentiated (G1) tumors among Md-BCs. The same disproportion was found in the group of undifferentiated tumors (G3), in which 32% of IDCs and 42% of MdBC were found.
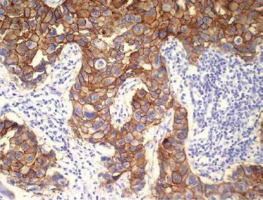
Most MdBC tumors (92%) were triple-negative, and 8% were HER2 positive (Figure 4). There were no ER and/or PR positive (luminal) BCs found in the studied group (Figure 5). The MdBC group included significantly more tumors with steroid hormone receptor negativity and no HER2 overexpression/gene amplification in contrast to the IDC group (ER-, 100% vs. 26%, p < 0.001; PR-, 100% vs. 29%, p < 0.001; HER2 0/1+, 92% vs. 82%, p = 0.004) (Table III, Figure 2). Data analysis showed that the highest percentage of IDC (68%) presented prominent steroid hormone receptor expression simultaneously demonstrating HER2 negativity (ER+, PR+, HER2 0/1+) (Figure 6).
Table III
Comparison of invasive ductal breast cancer (IDC) and medullary breast cancer (MdBC)
| Parameter | IDC (n = 856) | MdBC (n = 12) | P-value |
|---|---|---|---|
| Mean age [years] | 60.5 | 51.4 | – |
| Mean tumor size [cm] | 1.89 | 2.05 | – |
| Side: | |||
| Right breast | 404 (47.2) | 5 (41.67) | 0.703 |
| Left breast | 452 (52.8) | 7 (58.33) | |
| Tumor size (T-stage): | |||
| T1a | 19 (2.21) | – | < 0.001* |
| T1b | 72 (8.41) | – | |
| T1c | 332 (38.79) | 7 (58.33) | |
| T2 | 354 (41.36) | 5 (41.67) | |
| T3 | 26 (3.04) | – | |
| T4 | 53 (6.19) | – | |
| Lymph nodes (N-stage): | |||
| pN0 | 489 (57.13) | 10 (83.33) | < 0.001* |
| pN1 | 233 (27.21) | 2 (16.67) | |
| pN2 | 95 (11.10) | – | |
| pN3 | 39 (4.56) | – | |
| Tumor grade: | |||
| G1 | 73 (8.53) | – | 0.003* |
| G2 | 507 (59.23) | 7 (58.33) | |
| G3 | 276 (32.24) | 5 (41.67) | |
| Molecular subtypes: | |||
| Luminal | 641 (74.88) | – | < 0.001* |
| Triple negative | 99 (11.57) | 11 (91.67) | |
| HER2 overexpression | 116 (13.55) | 1 (8.33) | |
| Estrogen receptor status: | |||
| ER– | 219 (25.58) | 12 (100) | < 0.001* |
| ER+ | 637 (74.42) | – | |
| Progesterone receptor status: | |||
| PR– | 245 (28.62) | 12 (100) | < 0.001* |
| PR+ | 611 (71.39) | – | |
| HER2 status: | |||
| HER2 0/1+ | 702 (82) | 11 (91.67) | 0.004* |
| HER2 2+ | 51 (5.96) | – | |
| HER2 3+ | 103 (12.04) | 1 (8.33) | |
| TNM staging: | |||
| I | 236 (27.57) | – | < 0.001* |
| II | 365 (42.64) | 9 (75.0) | |
| III | 121 (14.14) | 3 (25.0) | |
| IV | 134 (15.64) | – | |
Figure 4
Example of immunohistochemical HER2 staining of triple-negative breast cancer, scored 0 (stained cells accounted for less than 10% of total tumor cells); the micrograph was taken with objective 20×
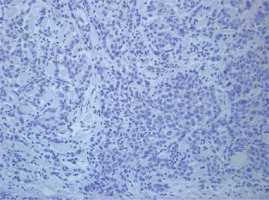
Figure 6
Strong, circumferential HER2 staining in > 30% of invasive ductal carcinoma (IDC) cells (3+) (original magnification, 20×)
According to the TNM staging criteria, in both investigated groups (MdBC, IDC), stage II tumors comprised the largest group (75%; 43%, respectively). Despite the presence of many features associated with histological malignancy, MdBCs were assessed only as stage II (75%) and III (25%) tumors. Among IDCs, 27% of tumors were assessed as stage I, 43% as stage II, 14% as stage III, and 16% were staged IV. A statistically significant difference was observed between distribution of clinical stages in both investigated groups (p < 0.001).
In order to determine overall MdBC prognosis, a 5-year follow-up of patients was conducted. In the present study we observed a 91% 5-year overall survival rate for MdBC patients.
Discussion
Breast cancer is one of the most prevalent female cancers worldwide. Even though the mortality rate decreases each year owing to earlier diagnoses and increasingly effective treatment, it remains the most common cause of cancer-related death in women [8]. It comprises many diverse subgroups, containing cancers with varying histopathological characteristics and clinical course. One of them is MdBC, seen rarely, but known for a favorable prognosis. It was defined for the first time in 1977, by Ridolfi et al. [9]. MdBC represents 1% to 7% of all diagnosed invasive BCs, which corresponds to the present study, which showed that MdBC comprises 1.07% of all invasive breast cancers. BC with medullary features is a histological diagnosis characterized by syncytial growth, well-circumscribed borders, and dense lymphocytic infiltration. MdBC usually has a soft consistency with a homogeneous gray and dense cut surface, but hemorrhage and central necrosis can be found in some cases. Histologically, medullary tumors consist of large cells. Most MdBCs, despite their worrisome cytologic and histologic features, triple-negativity, and high mitotic activity, have a favorable prognosis [10]. Invasive ductal breast carcinoma stands in contrast to MdBC. Invasive ductal subtype of BC is the most prevalent histological subtype BC in the Polish and European population as well, and is responsible for significant breast cancer mortality [11]. Considering the fact that IDC is the most frequent type of breast malignancy while MdBC is among the rarest subtypes, it is essential to provide a comparison of these breast cancers, especially focused on histopathological features and patients’ overall survival.
The average age of MdBC patients in the present study was 54.5 years, which is slightly more than in available analyses (usually patients’ mean age ranges from 45 to 54 years) [12]. Women under 40 years of age are generally defined as young in the contemporary reports, and those under 35 years of age as very young. The proportion of very young patients (< 35 years old) was 16.7% in the present study, similarly to results of a study conducted by Park et al. which revealed that 13.5% of analyzed patients were under 35 [12]. That fact proves that MdBC is especially a problem in the youngest group of patients, and that MdBC patients are usually younger than those who suffer from different types of BC, including IDC [13]. The average age of IDC patients in the present study was 60.5 years, which corresponds to the available data. It has also been proven that occurrence of BC at earlier ages is usually related to BRCA1 and/or BRCA2 mutations [13, 14]. According to the published data, MdBC more often can be found in African-American and Japanese women compared to women of the white race. Moreover, MdBC diagnosis is extremely rare in males, representing less than 0.5% of even such rare male breast cancer [15].
Previous studies presented controversial results regarding MdBC tumor size. Flucke et al. and Wang et al. reported smaller tumor diameter in MdBC compared to the IDC [16, 17]. On the other hand, Oh et al. found that MdBC had larger tumor size than IDC (p < 0.001) [18]. The present study showed that MdBCs are slightly larger than IDC tumors (2.05 cm vs. 1.89 cm, respectively) (Table III).
In the previous studies, regional lymph nodes metastasis was not observed in most of the MdBC patients. In the present analysis the majority of patients (83%) were assessed as pN0 and the remaining 17% of patients were pN1. High lymph node involvement (pN3, pN4) has not been observed, which reflects positively on the overall survival (OS) and recurrence-free survival (RFS) time [12, 19–22]. The incidence of nodal metastasis is usually lower than in other BCs, especially IDC [21]. Taking into account local invasion, it seems that IDC tumors are more aggressive than MdBC. Flucke et al. also proved that MdBC patients had a notably higher node-negative rate compared to those with IDC (75% vs. 48%, respectively; p = 0.0014) [16]. This conclusion may be related to the histopathological characteristics of MdBC, which include pronounced lymphocytic infiltration particularly with CD3, CD8, granzyme-B positive and TIA-1 lymphocytes. The other explanation may be the fact of a dissimilar immune response to cancer cells in MdBC than in other tumors, such as the presence of IgG, the absence of IgA, dense infiltration of plasma cells, and expression of tumor-specific antigens, e.g. HLA-DR, ganglioside D3, and β-actin [12, 23, 24]. These processes might be engaged in the tumor spread control and general invasiveness. By blocking the metastatic potential they improve the MdBC prognosis. Rare regional lymph node metastasis is not only a characteristic feature of MdBC, but seems to be the crucial prognostic factor and a sign of a cancer invasiveness too. The essential importance of lymph node involvement in MdBC has been reported in numerous studies [14, 25, 26]. Martinez et al. reported that 10-year survival rates in MdBC with and without regional lymph node involvement were 67.5% and 81.9%, respectively [14]. Furthermore, Ridolfi et al. reported that patients with regional lymph node metastasis to < 3 lymph nodes did not die significantly earlier from the disease [9]. In the present study, the 5-year survival rate was 91%, and a significant difference between pN0 and pN1 MdBCs was not observed.
Hormonal receptor expression profile (ER, PR, and HER2) is widely known as a predictive prognostic factor. Hormonal status also implies the choice of the therapeutic strategy. It is also proven that MdBC is characterized by a lower incidence of ER, PR and HER2 expression. In the present study all MdBCs were ER- and PR-negative, whereas 92% of them were HER2-negative (HER2 0/1+). Therefore it was observed that the vast majority (92%) of MdBCs showed triple-negativity, similarly to the previous studies [1, 14, 27]. As mentioned above, the molecular subtype of BC is one of the most significant factors influencing the clinical course. It is widely known that both triple-negative and HER2 overexpressing subtypes predict a serious prognosis. In the present study, patients with a triple-negative disease were younger at the time of the primary diagnosis compared to those with other molecular BC types. When comparing all breast cancer histological subtypes, the same observation can be made. MdBC patients were on average at least 5 years younger than patients with any other histological type of BC. In the present analysis, the triple-negative breast cancer ratio was significantly higher in the MdBC compared to the IDC group (p < 0.001).
Many analyses have reported a much more favorable prognosis for MdBC patients compared to those suffering from IDC [28]. Xue et al. reported that the 2-year RFS and OS rates for triple-negative IDCs were 79% and 82%, respectively [29]; whereas Zhaohiu et al. reported that the 2-year RFS and OS rates for triple-negative MdBC were 98.2% and 99.1%, respectively [28]. A statistically significant difference was also detected between MdBC and IDC patients with respect to the 5-year RFS (94.2% vs. 86.3%, p = 0.008) [18]. Several years later Cao et al. concluded that MdBC in Chinese women was characterized by a less aggressive clinical outcome and a better prognosis than IDC also after 10 years of follow-up [30]. Huober et al. reported that 14-year distant RFS and OS percentages for MdBC and IDC tumors were 76%, 64% and 66%, 57%, respectively [31]. In other words, although the MdBC patients predominantly exhibit a triple-negative molecular phenotype, their clinical outcome is better compared to the IDC patients [32, 33]. This finding proves that the triple-negative molecular phenotype of BC is an insufficient factor for predicting overall prognosis as it had been thought for many years. The favorable overall prognosis for MdBC patients might be clarified through gene expression profiling. Vincent-Salomon et al. reported that cytokeratin 5/6 was expressed significantly more strongly in MdBC than in any other breast cancer histological subtype [34, 35]. Moreover, Bertucci et al. observed an extremely effective host immune response, upregulated expression of metastasis-inhibiting agents, and enhanced cancer cell apoptosis as histological features associated with better prognosis [36]. High frequency of remarkable inflammation and uncommon fibrosis regions are also biological features associated with a good prognosis [37, 38].
In conclusion, the low level of MdBC diagnosis is suspected to be caused by the absence of unequivocal histological and immunohistochemical diagnostic criteria. There are some features making MdBC recognition easier, such as syncytial pattern of tumor growth, lymphocyte infiltration, absence of tubular structures, infrequent necrosis foci, high mitotic rate, enriched cytoplasm and a very high level of genome instability, but they are not specific enough. Despite many aggressive pathological features of MdBC, its clinical outcome is much more favorable than for any other breast cancer type. This study proves that IDCs and Md-BCs are completely different and independent types of the breast malignancy. In the vast majority of cases, MdBC diagnosis is associated with the triple-negative phenotype. It should be remembered that triple-negativity is usually associated with poor prognosis, but the MdBC group seems to be an important exception. Since triple-negativity can be related to both IDC and MdBC tumors, it is necessary to describe new markers and prognostic factors for this rare type of BC in order to enhance the accuracy of the diagnoses made and the effectiveness of oncological treatment.