Bladder cancer (Bca) is a malignant condition that affects the urinary bladder and typically originates from the urothelial cells that line the bladder. Muscle-invasive bladder cancer (MIBC) has a relatively high risk of progression and metastasis [1]. The global annual number of new cases of bladder cancer exceeds 500,000 [2]. This disease primarily affects adults, with the majority of diagnoses occurring in individuals over the age of 55. Symptoms commonly experienced by patients include gross or microscopic hematuria (the presence of blood in the urine), which can be quite distressing [3].
In recent years, antihypertensive drugs, as a core strategy for the primary and secondary prevention of cardiovascular diseases (CVDs), have formed a multilevel evidence chain because of their benefits in reducing all-cause mortality and major adverse cardiovascular events (MACEs). A Global Burden of Disease (GBD) study revealed that hypertension remains the leading modifiable risk factor for death worldwide, and systematic blood pressure-lowering treatment can significantly reverse this trend [4]. Against this backdrop, the European Society of Cardiology (ESC) and the American Heart Association/American College of Cardiology (AHA/ACC) have clearly set blood pressure control (systolic blood pressure < 130 mm Hg) as the primary goal of hypertension management, emphasizing the need to develop individualized plans on the basis of the characteristics of drug classes and patient features [5]. A substantial amount of evidence-based support has been obtained for the role of antihypertensive drugs in reducing the risk of cardiovascular diseases and all-cause mortality. The renin–angiotensin–aldosterone system (RAAS) inhibitors include ACEIs and ARBs. Both the RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) and IDNT (Irbesartan Diabetic Nephropathy Trial) studies have shown that losartan and irbesartan demonstrate cardiovascular (CV) benefits, reducing the incidence of cardiovascular-related death, congestive heart failure, myocardial infarction, and cerebrovascular events. Furthermore, in a real-world study of elderly hypertensive patients in Italy, current users of calcium channel blockers significantly reduced the risk of CV events, hospitalization, and mortality [6]. A systematic review from the Cochrane Library [7] also revealed that, compared with placebo, β-blockers were associated with a lower total incidence of CVD.
Numerous epidemiological observational studies have explored the association between the use of antihypertensive medications and the risk of bladder cancer, but the findings have been inconsistent. For example, a case-control study from Taiwan Province, China [8], reported a significant association between the use of diuretics and any type of urinary tract cancer; however, a population-based case-control study from Los Angeles [9] reported that the use of high doses of diuretics was not associated with the occurrence of Bca. These conflicting results may be related to methodological biases in traditional pharmacoepidemiologic studies; for example, if confounding factors such as smoking, alcohol consumption, diet, and obesity of study participants are not adjusted for, residual confounding may arise, thereby affecting the reliability of the study outcomes.
Drug-target Mendelian randomization (MR), a research method that utilizes genetic approaches to investigate the causal relationship between drug targets and disease outcomes, can avoid the confounding biases present in traditional pharmacoepidemiologic studies, thus allowing for inferences about whether drug targets have a causal effect on diseases. Therefore, the objective of this study was to conduct a comprehensive drug-target-based MR analysis to systematically assess the causal relationships between various classes of antihypertensive medications (Supplementary Table SII) and bladder cancer, with the study population including individuals of European and East Asian descent.
Methods
This study conforms to the MR_STROBE reporting guidelines (Supplementary Table SI) and is predicated on the three fundamental assumptions of MR (Supplementary Figure S1). A schematic representation of the study’s methodology is depicted in Supplementary Figure S1. The data sources utilized in this research are specified in Supplementary Table SIII. Primary analysis of bladder cancer outcomes in Europeans was conducted via the FinnGen Biobank dataset, with replication analysis performed by the Neale Lab. For East Asians, the bladder cancer outcome study was based on data from the China Kadoorie Biobank (CKB).
In accordance with the previously published MR study [10], for European participants, the present study utilized the largest GWAS summary dataset on bladder cancer, which was freely accessible from the FinnGen Biobank. In total, the dataset includes 2,682 cases and 26,795 controls related to Bca outcomes, predominantly identified through ICD-10 codes (ICD-O-3). During the replication analysis, for bladder cancer, the genetic data are from the Neale laboratory, which conducted a GWAS on 361,194 Europeans (Ncase = 1,554, Ncontrol = 359,640), identifying a total of 10,267,743 SNPs. The genetic data for bladder cancer in East Asian populations are from the China Kadoorie Biobank (CKB) [11], which performs a GWAS on 75,744 Chinese populations. More details are summarized in Supplementary Table SIII.
Supplementary Tables SIV and SV summarize the genetic proxy information for antihypertensive medications in European and East Asian populations, respectively. Supplementary Table SVI includes antihypertensive drugs that are significantly negatively associated with CAD and were selected for further analysis to explore their causal relationship with Bca. Notably, in the analysis of East Asian populations, following the harmonization of data for genetically predicted exposure (BBs) and the outcome of bladder cancer, it was observed that the associated single-nucleotide polymorphisms (SNPs) (rs10885532, rs180912, and rs78006240) presented palindromic structures and intermediate allele frequencies. Consequently, genetically predicted exposures (BBs) in East Asian populations were excluded from further analysis.
For statistical analysis, the IVW approach was used as the primary method for MR analysis. In this MR study, we conducted multiple tests (evaluating various antihypertensive medicines) with Bonferroni correction. In MR studies, the inverse variance weighted (IVW) method is a widely utilized statistical approach for estimating causal effects [12]. This method operates by calculating a weighted average of the effect estimates from multiple genetic variants (e.g., single nucleotide polymorphisms – SNPs), where the weights correspond to the inverse of the variance (i.e., the squared standard errors) of each genetic variant’s effect estimate. Specifically, the IVW approach employs a regression model that excludes an intercept term and incorporates the inverse of the outcome variance as weights during fitting. This methodology assumes that all genetic variants serve as valid instrumental variables (IVs) and satisfy three core MR assumptions: (1) the relevance assumption, (2) the independence assumption, and (3) the exclusion restriction assumption. When these assumptions hold, the IVW method provides consistent and unbiased estimates of the causal effect.
Sensitivity analysis in MR studies involves reassessing the robustness of the causal relationship. This includes methods such as the Cochran’s Q test to assess heterogeneity and horizontal pleiotropy of IVs [13]. Confounding analysis in MR studies ensures that genetic instruments are not associated with other factors (such as smoking alcohol, drinking, diet, and obesity) that could influence the outcome. This investigation typically involves the use of reverse MR methods [14].
Further methodological details are described in the Supplementary Methods.
Results
In the primary MR analysis, the MR results indicated no statistically significant associations between genetically predicted BBs, CCBs, LDs and Vas and a high risk of Bca in European populations, utilizing the outcome dataset from the FinnGen Biobank (EUR_FinnGen Biobank: PIVW_BBs =0.4500, PIVW_CCBs = 0.5791, PIVW_LDs = 0.1027, and PIVW_Vas = 0.3672) (Figure 1 A and Supplementary Table SVII). The results of four additional MR analyses are presented in Supplementary Table SVII.
Figure 1
A – Forest plot map of Mendelian randomization (MR) analysis, utilizing genetic proxies for four classes of antihypertensive drugs, demonstrating their association with the risk of bladder cancer. The primary outcome is based on data from the FinnGen Biobank. B – Forest plot map of MR analysis, using genetic proxies for four classes of antihypertensive drugs, illustrating their association with the risk of bladder cancer. The secondary outcome is derived from the Neale lab dataset C – Leave-one-out analysis was conducted for the following antihypertensive drug classes: (1) β-adrenoceptor blockers, (2) calcium channel blockers, (3) loop diuretics, and (4) vasodilator antihypertensives. This analysis was applied to both the primary analysis of bladder cancer risk in the FinnGen Biobank and the replication analysis in the Neale lab C – Leave-one-out analysis was conducted for the following antihypertensive drug classes: (1) β-adrenoceptor blockers, (2) calcium channel blockers, (3) loop diuretics, and (4) vasodilator antihypertensives. This analysis was applied to both the primary analysis of bladder cancer risk in the FinnGen Biobank and the replication analysis in the Neale lab C – Leave-one-out analysis was conducted for the following antihypertensive drug classes: (1) β-adrenoceptor blockers, (2) calcium channel blockers, (3) loop diuretics, and (4) vasodilator antihypertensives. This analysis was applied to both the primary analysis of bladder cancer risk in the FinnGen Biobank and the replication analysis in the Neale lab. D – Confounding analysis was conducted to assess the impact of genetic proxies for β-adrenoceptor blockers, specifically focusing on the inclusion or exclusion of the rs1801253 (rs1801253: cigarette smoking), on the association with bladder cancer risk in the replication data from the Neale lab
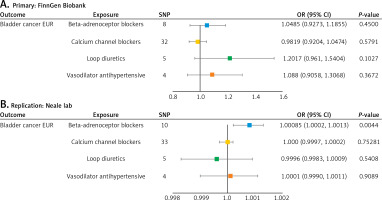



In subsequent replication analysis, interestingly, a harmful effect of genetically proxied BB drugs was observed in the European Bca population from the Neale laboratory dataset (ORIVW_BBs = 1.0008, 95% CI (1.0002, 1.0013), PIVW_BBs = 0.0044). For other antihypertensive drugs, including CCBs, LDs, and Vas, no significant associations with the risk of European Bca populations from the Neale laboratory dataset were observed (EUR_Neale laboratory: PIVW_CCBs = 0.7528, PIVW_LDs = 0.5408, and PIVW_Vas = 0.9089) (Figure 1 B).
LOO analysis further confirmed the stability of the aforementioned findings, which were derived from both the primary and replication analyses (Figure 1 C).
In the confounding analysis, one pleiotropic SNP (rs1801253 pertaining to smoking in the beta-adrenoceptor blockers, NHGRI-EBI GWAS catalog in ontology accessions: EFO:0006336 and referenced by PMID:29455858) confirmed by Ensemble Asian (https://asia.ensembl.org/) (outcome: bladder cancer risk in the replication dataset from the Neale laboratory) was identified, and following the elimination of this specific SNP, the findings remained in alignment with previously identified significant relationships (PIVW = 0.0305) (Figure 1 D). However, this statistically significant correlation did not successfully pass the Bonferroni correction test (the threshold of PBonferroni_correction was 0.0125) (Figure 1 D). Therefore, further validation through confounding analysis and Bonferroni correction revealed that this statistically significant genetic association was not confirmed in the European bladder cancer population from the Neale laboratory dataset. The current evidence from MR studies still does not substantiate the association between genetically proxied BB drugs and bladder cancer in the European population.
Furthermore, in the East Asian population, no statistically significant correlation was found between genetically proxied CCBs or LDs and a higher risk of Bca (PIVW_CCBs = 0.6493 and PWald_ratio_LDs = 0.5815) (Supplementary Table SVIII).
Additional analysis confirmed that there was no heterogeneity (Pheterogeneity > 0.05), horizontal pleiotropy (PIntercept > 0.05 and PMRPRESSO_Global_Test > 0.05) (Supplementary Table SIX), or reverse causality in our MR study involving European populations (PSteiger_filtering < 0.05 and correct causal direction = True) (Supplementary Table SIX). However, owing to the limited number of SNPs included, the pleiotropy of LDs and CCBs in the East Asian population could not be detected. Additionally, the heterogeneity of LDs could not be calculated because of the insufficient number of SNPs included.
Discussion
The potential carcinogenic effects of antihypertensive drugs add to the psychological burden of patients with cardiovascular diseases taking medication. Cardiovascular diseases are the leading cause of death worldwide, and discontinuing necessary antihypertensive treatment due to fear of the potential carcinogenic effects of antihypertensive drugs may increase the risk of death for cardiovascular patients [15]. However, there is currently inconsistency in the results regarding the correlation between the use of antihypertensive drugs and cancer risk. Wang et al. [16] conducted a population-based cohort study in Shanghai, China, to investigate the relationship between antihypertensive drugs and cancer risk. The study revealed that calcium channel blockers (CCBs) were moderately associated with increased total cancer risk. In contrast, Copland et al. [17] conducted a network meta-analysis comparing the effects of different antihypertensive drugs with those of placebos and reported that no excess cancer risk was associated with any type of antihypertensive drug.
In previous observational studies, patients used more than one antihypertensive drug over a period of time, making it impossible to determine the impact of a single antihypertensive drug on bladder cancer. In addition, previous observational studies have shortcomings, such as recall bias, short follow-up periods (less than 10 years), and poor patient medication adherence, which still results in inconsistencies in the research findings regarding the use of antihypertensive drugs and the risk of bladder cancer [18].
This drug-targeted MR study, which uses SNPs associated with various antihypertensive drugs as IVs, can investigate the associations between the lifelong use of a single drug target of antihypertensive drugs and the risk of bladder cancer, avoiding the influence of confounding factors and reverse causality. The results of this study show that these antihypertensive drugs do not have a genetic causal relationship with the risk of bladder cancer. This result has been verified in both European and Asian populations.
Interestingly, preclinical experimental studies have shown that β-blockers (BBs) can regulate inflammation and block norepinephrine, which is closely related to cancer promotion and metastasis in the tumor environment, as well as cAMP-dependent intracellular signal transduction, to reduce tumor proliferation and migration [19]. However, previous observational studies revealed that the positive association between BBs and the risk of bladder cancer disappeared after adjusting for the confounding factor of smoking. The results of this Mendelian randomization (MR) study are similar to those of Xie et al. [20], where in the validation dataset (data from the Neale laboratory), after removing the pleiotropic SNP (rs1801253) closely related to smoking, it was found that genetically proxied BBs drugs and the risk of bladder cancer occurrence in the European population were not significantly associated. Therefore, the combination of previous clinical studies and the results of this MR study suggest that the significant associations between BBs and the risk of bladder cancer may be attributed to unadjusted risk factors. Similarly, Jiang et al. [9], on the basis of a case‒control study of the Los Angeles population, noted that the treatment of hypertension, including the use of diuretics, may have some interaction with the risk of bladder cancer, and this interaction may be influenced by individual genetic background. Therefore, when the relationship between diuretics and the risk of bladder cancer is considered, individual genetic susceptibility and lifestyle factors, such as smoking, need to be considered.
Previously, Fan et al. [21] applied drug-targeted MR methods to explore the correlation between genetic proxies for calcium channel blockers (CCBs) and cancer risk. The study revealed that under the Bonferroni correction threshold (p = 0.003), there was no significant correlation between genetic proxies for CCBs and the risk of any type of tumor. This study included data from two specific European bladder cancer databases (FinnGen and Neale lab) and once again confirmed that there was no significant correlation between genetic proxies for CCBs and the risk of bladder cancer. Furthermore, the results of this study further revealed that, in addition to the European bladder cancer population, no associations were found between genetic proxies for calcium channel blockers and bladder cancer in the East Asian population, which further supplements the previous MR study results of Fan et al. [21].
The majority of participants were of European or East Asian descent, limiting the applicability of the results to other ethnic groups [22]. Second, in the East Asian population, the limited number of included single-nucleotide polymorphisms (SNPs) may result in insufficient statistical power to detect pleiotropy and heterogeneity for LDs and CCBs. This limitation hampers our in-depth understanding of the potential associations between these medications and bladder cancer in East Asian populations.
In conclusion, this study revealed no evidence supporting a genetic causal relationship between antihypertensive medications and bladder cancer in both European and Asian population while confirming their irreplaceable role in reducing cardiovascular risk and all-cause mortality.