Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
Development and verification of a novel disulfidptosis-related lncRNA prognostic model for predicting the immune environment and treatment of breast cancer
1
Department of Oncology, The First Affiliated Hospital of Anhui Medical University, Anhui, China
2
Department of Radiation Oncology, The First Affiliated Hospital of Anhui Medical University, Anhui, China
3
Department of Intensive Care Unit, West District of The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Anhui, China
These authors had equal contribution to this work
Submission date: 2024-09-26
Final revision date: 2025-03-05
Acceptance date: 2025-03-17
Online publication date: 2025-04-25
Corresponding author
Jiqing Hao
Department of Radiation Oncology, The First Affiliated Hospital of Anhui Medical University Anhui, China
Department of Radiation Oncology, The First Affiliated Hospital of Anhui Medical University Anhui, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Breast cancer is the leading cause of cancer-related death in women. Disulfidptosis is a recently identified type of cell death that may offer new opportunities for cancer treatment. However, it is uncertain whether disulfidptosis-related lncRNAs (DRlncRNAs) are associated with BRCA.
Material and methods:
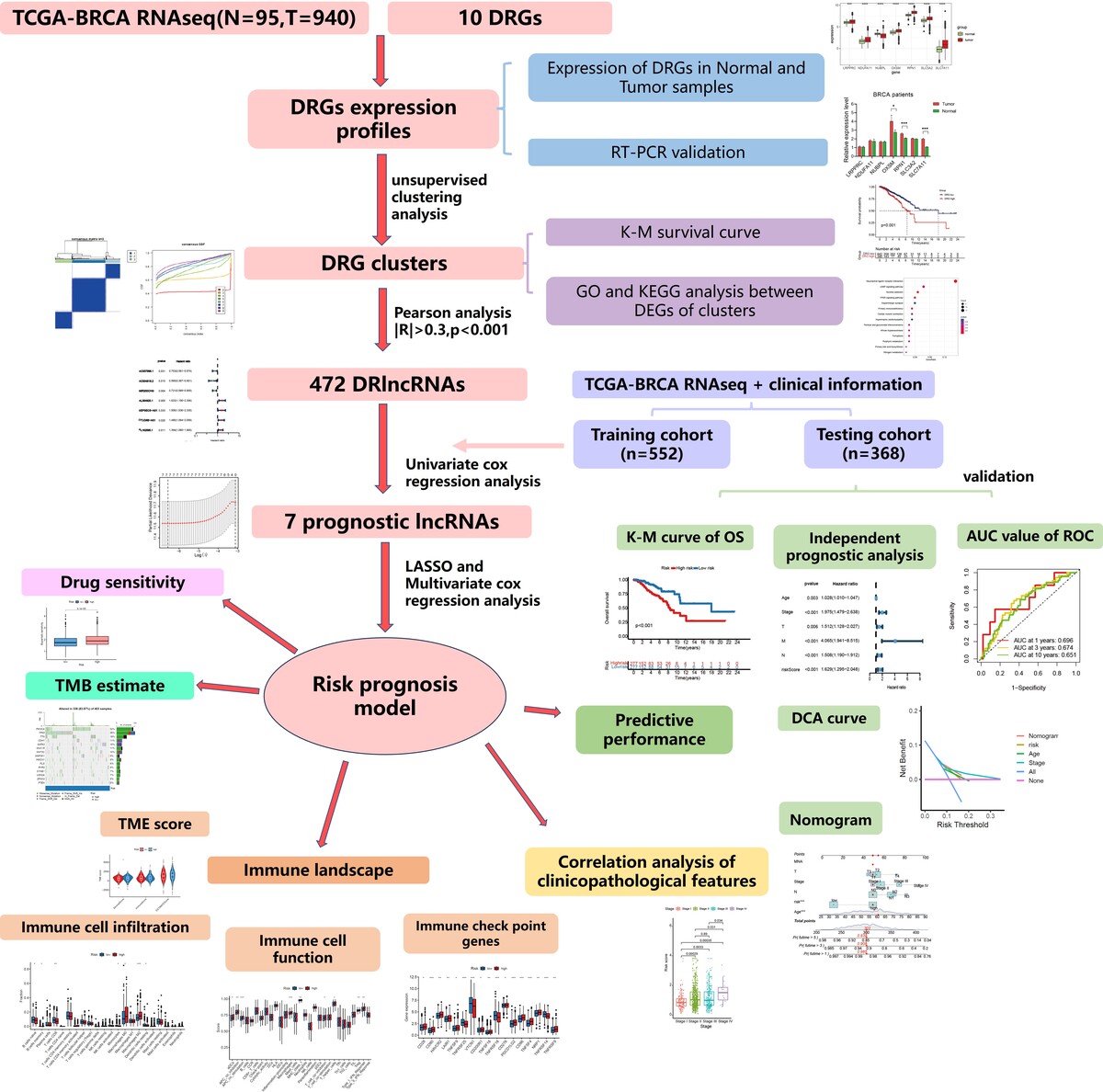
We first evaluated the expression of disulfidptosis-related genes (DRGs) by RT-PCR. We then identified DRlncRNAs using Pearson’s correlation, followed by univariate regression to select prognosis-related genes. LASSO regression and multivariate Cox regression were used to construct a prognostic model, and ROC curves were used to evaluate the model’s predictive performance. We compared infiltration of various immune cells and expression of immune checkpoint genes between risk groups. Maftools was employed to analyze the tumor mutation burden (TMB) of patients. Finally, the pRRophetic package was used to analyze the sensitivity of patients to anticancer drugs.
Results:
We found that OXSM, RPN1, SLC3A2, and SLC7A11 showed increased expression levels in tumor tissues compared to normal tissues. We then constructed and validated a prognostic model (AC007996.1, AC004816.2, MIR200CHG, AL354920.1). Patients in the high-risk group had significantly reduced percentages of naive B cells and CD8+ T cells, and higher expression levels of immune checkpoint-related genes compared to patients in the low-risk group, suggesting immune escape ability of the high-risk group. Patients in the high-risk group had a higher TMB. Finally, patients in the high-risk group had higher IC50 values for many targeted agents, suggesting poor drug sensitivity.
Conclusions:
We identified DRG expression in breast cancer, and constructed a prognostic model predicting the prognosis, the immune microenvironment, TMB, and drug sensitivity.
Breast cancer is the leading cause of cancer-related death in women. Disulfidptosis is a recently identified type of cell death that may offer new opportunities for cancer treatment. However, it is uncertain whether disulfidptosis-related lncRNAs (DRlncRNAs) are associated with BRCA.
Material and methods:
We first evaluated the expression of disulfidptosis-related genes (DRGs) by RT-PCR. We then identified DRlncRNAs using Pearson’s correlation, followed by univariate regression to select prognosis-related genes. LASSO regression and multivariate Cox regression were used to construct a prognostic model, and ROC curves were used to evaluate the model’s predictive performance. We compared infiltration of various immune cells and expression of immune checkpoint genes between risk groups. Maftools was employed to analyze the tumor mutation burden (TMB) of patients. Finally, the pRRophetic package was used to analyze the sensitivity of patients to anticancer drugs.
Results:
We found that OXSM, RPN1, SLC3A2, and SLC7A11 showed increased expression levels in tumor tissues compared to normal tissues. We then constructed and validated a prognostic model (AC007996.1, AC004816.2, MIR200CHG, AL354920.1). Patients in the high-risk group had significantly reduced percentages of naive B cells and CD8+ T cells, and higher expression levels of immune checkpoint-related genes compared to patients in the low-risk group, suggesting immune escape ability of the high-risk group. Patients in the high-risk group had a higher TMB. Finally, patients in the high-risk group had higher IC50 values for many targeted agents, suggesting poor drug sensitivity.
Conclusions:
We identified DRG expression in breast cancer, and constructed a prognostic model predicting the prognosis, the immune microenvironment, TMB, and drug sensitivity.
REFERENCES (49)
1.
Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022; 72: 409-36.
2.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7-33.
3.
Badowska-Kozakiewicz AM, Budzik MP, Liszcz A, et al. Clinicopathological factors associated with novel prognostic markers for patients with triple negative breast cancer. Arch Med Sci 2019; 15: 1433-42.
4.
Liu YB, Gao XT, Huang LY, Liu XL. Clinicopathological characteristics and prognostic factors in invasive micropapillary carcinoma of the breast. Arch Med Sci 2024; 20: 428-35.
5.
Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta 2014; 1839: 1097-109.
6.
Yin Z, Wang J, Zhu C, Xu C, Fang J, Li Q. Identification and verification of a novel disulfidptosis-related lncrnas prognostic signature to predict the prognosis and immune activity of head and neck squamous carcinoma. Iran J Public Health 2024; 53: 2328-40.
7.
Lin J, Lin N, Zhao W. Development and validation of a prognostic nomogram for lower-grade glioma based on an autophagy-related lncRNA signature. Arch Med Sci DOI: https://doi.org/10.5114/aoms/1....
8.
Lu R, Zhang J, Zhang W, et al. Circulating HOTAIR expression predicts the clinical response to neoadjuvant chemotherapy in patients with breast cancer. Cancer Biomark 2018; 22: 249-56.
9.
Özgür E, Ferhatoğlu F, Şen F, Saip P, Gezer U. Circulating lncRNA H19 may be a useful marker of response to neoadjuvant chemotherapy in breast cancer. Cancer Biomark 2020; 27: 11-7.
10.
Elhasnaoui J, Miano V, Ferrero G, et al. DSCAM-AS1-driven proliferation of breast cancer cells involves regulation of alternative exon splicing and 3’-end usage. Cancers (Basel) 2020; 12: 1453.
11.
Jiang W, Pan S, Chen X, Wang ZW, Zhu X. The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol Cancer 2021; 20: 116.
12.
Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022; 375: 1254-61.
13.
Liu X, Nie L, Zhang Y, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol 2023; 25: 404-14.
14.
He WP, Chen YY, Wu LX, Guo YY, You ZS, Yang GF. A novel necroptosis-related lncRNA signature for predicting prognosis and anti-cancer treatment response in endometrial cancer. Front Immunol 2022; 13:.
15.
Cui H, Lian J, Xu B, et al. Identification of a bile acid and bile salt metabolism-related lncRNA signature for predicting prognosis and treatment response in hepatocellular carcinoma. Sci Rep 2023; 13: 19512.
16.
Wang J, Zheng Q, Jian J, et al. Construction of a disulfidptosis-associated lncRNA signature to predict prognosis in bladder cancer. Transl Androl Urol 2024; 13: 2705-23.
17.
Zheng Y, Lin Y, Zhang Y, Liu S, Yang Y, Huang W. Determining new disulfidptosis-associated lncRNA signatures pertinent to breast cancer prognosis and immunological microenvironment. Transl Cancer Res 2024; 13: 5815-29.
18.
Liu S, Zheng Y, Li S, et al. Integrative landscape analysis of prognostic model biomarkers and immunogenomics of disulfidptosis-related genes in breast cancer based on LASSO and WGCNA analyses. J Cancer Res Clin Oncol 2023; 149: 16851-67.
19.
Chen X, Yang C. A novel disulfidptosis-related lncRNAs prognostic signature for prognosis predicting and immune microenvironment characterization in breast cancer. Curr Med Chem 2024. doi: 10.2174/0109298673294711240405090150.
20.
Wu J, Cai Y, Zhao G. Identification of disulfidptosis-related clusters and construction of a disulfidptosis-related gene prognostic signature in triple-negative breast cancer. Heliyon 2024; 10: e33092.
21.
Tang L, Wei D, Xu X, et al. Long non-coding RNA MIR200CHG promotes breast cancer proliferation, invasion, and drug resistance by interacting with and stabilizing YB-1. NPJ Breast Cancer 2021; 7: 94.
22.
Capper CP, Rae JM, Auchus RJ. The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm Cancer 2016; 7: 149-64.
23.
Cramer DW, Harlow BL, Willett WC, et al. Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet 1989; 2: 66-71.
24.
Bao Y, Wang L, Shi L, et al. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell Mol Biol Lett 2019; 24: 38.
25.
Yarla NS, Bishayee A, Sethi G, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol 2016; 40-41: 48-81.
26.
Fanale D, Amodeo V, Caruso S. The interplay between metabolism, PPAR signaling pathway, and cancer. PPAR Res 2017; 2017: 1830626.
27.
Wang B, Wu L, Chen J, et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther 2021; 6: 94.
28.
Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013; 501: 346-54.
29.
Pan G, Xie H, Xia Y. Disulfidptosis characterizes the tumor microenvironment and predicts immunotherapy sensitivity and prognosis in bladder cancer. Heliyon 2024; 10: e25573.
30.
Liu C, Somasundaram A, Manne S, et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat Immunol 2020; 21: 1010-21.
31.
Chuckran CA, Liu C, Bruno TC, Workman CJ, Vignali DA. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J Immunother Cancer 2020; 8: e000967.
32.
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci 2019; 110: 2080-9.
33.
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol 2020; 353: 104119.
34.
Wang Y, Smith W, Hao D, He B, Kong L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol 2019; 70: 459-66.
35.
Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells 2020; 9: 1299.
36.
Wei Z, Zhang X, Yong T, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun 2021; 12: 440.
37.
Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer (Dove Med Press) 2015; 7: 111-23.
38.
Zhang Y, Xiong S, Liu B, et al. Somatic Trp53 mutations differentially drive breast cancer and evolution of metastases. Nat Commun 2018; 9: 3953.
39.
Badve SS, Gökmen-Polar Y. TP53 status and estrogen receptor-beta in triple-negative breast cancer: company matters. J Natl Cancer Inst 2019; 111: 1118-9.
40.
Pham TT, Angus SP, Johnson GL. MAP3K1: genomic alterations in cancer and function in promoting cell survival or apoptosis. Genes Cancer 2013; 4: 419-26.
41.
Avivar-Valderas A, McEwen R, Taheri-Ghahfarokhi A, et al. Functional significance of co-occurring mutations in PIK3CA and MAP3K1 in breast cancer. Oncotarget 2018; 9: 21444-58.
42.
Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012; 486: 405-9.
43.
Fuso P, Muratore M, D’Angelo T, et al. PI3K inhibitors in advanced breast cancer: the past, the present, new challenges and future perspectives. Cancers (Basel) 2022; 14: 2161.
44.
Garrido-Castro AC, Saura C, Barroso-Sousa R, et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res 2020; 22: 120.
45.
Pistilli B, Pluard T, Urruticoechea A, et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat 2018; 168: 357-64.
46.
Schade AE, Perurena N, Yang Y, et al. AKT and EZH2 inhibitors kill TNBCs by hijacking mechanisms of involution. Nature 2024; 635: 755-63.
47.
Stover DG, Gil Del Alcazar CR, Brock J, et al. Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer 2018; 4: 10.
48.
Teo ZL, O’Connor MJ, Versaci S, et al. Combined PARP and WEE1 inhibition triggers anti-tumor immune response in BRCA1/2 wildtype triple-negative breast cancer. NPJ Breast Cancer 2023; 9: 68.
49.
Ji D, Luo Y, Wang J, et al. CDK4/6 inhibitors, PI3K/mTOR inhibitors, and HDAC inhibitors as second-line treatments for hormone receptor-positive, HER2-negative advanced breast cancer: a network meta-analysis. BMC Cancer 2023; 23: 805.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.