Hepatocellular carcinoma (HCC) is one of the most common cancers and the second leading cause of cancer death worldwide [1]. In 2015, about 4.3 million patients suffered from HCC and 2.8 million people died of the disease in China. Approximately 70% of HCC patients who underwent liver transplantation or surgical resection still faced a higher risk of recurrence in 5 years. In addition, the 5-year overall survival (OS) rate of HCC is considerably low, which forebodes a poor prognosis. This condition prompted researchers worldwide to focus on the mechanism of HCC and identify new clinical treatments for patients [2]. In this study, a case of advanced liver cancer targeted therapy combined with immunotherapy plus local radiotherapy and transarterial chemoembolization (TACE) was followed up for more than 36 months. Moreover, the pathogenesis, treatment, and prognosis of this disease are discussed in reference to the relevant literature.
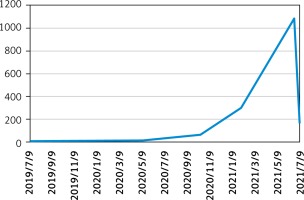
Medical history: The patient is male, and aged 54 years. In August 2019, multiple solid nodules were found in the liver by abdominal B-ultrasound, and the possibility of liver cancer was not ruled out. The patient did not pay attention to this finding, and initiated herbal treatment by himself. However, the effect was not good. In May 2019, due to right chest pain, chest computed tomography (CT) plain scan and enhanced scan in an external hospital showed that the bones on the right side of the sternum were destroyed, and metastasis was considered. The patient has a history of chronic hepatitis B. Personal history, marital history, and family history were not significant. In July 2019, the patient went to the Affiliated Hospital of Guangdong Medical University, Guangdong Province, China. Chest and upper abdomen CT: HCC (42 mm × 20 mm × 29 mm) in the upper segment of the right anterior lobe of the liver and the formation of multiple sub-foci in the liver were identified (Figures 1, A1-2). Moreover, suspected tumor thrombus formation was found on the upper branch of the portal vein on the right anterior lobe of the liver. In addition, the bones on the left side of the lumbar 2 vertebral body were destroyed, and there were soft tissue shadows next to the right side of the sternum (Figure 1, parasternal lesion A3, lumbar paralumbar A4). Whole body bone scintigraphy suggested that the right side of the manubrium and the second lumbar vertebra had a high possibility of metastases. The AFP was 7.01 μg/l. For a definite diagnosis, a liver biopsy was performed on July 10, 2019, and the postoperative pathology showed HCC (Figure 2). At that time, the diagnosis was primary liver cancer with multiple bone metastases (stage IV). Combined with the patient’s situation and the guidelines of multidisciplinary diagnosis and treatment (MDT), it is recommended to perform radiotherapy for bone metastases, concurrent or sequential molecular targeted/immunotherapy. Lumbar spine intensity-modulated radiotherapy was started on July 18, 2019. The specific dose was PTV40GY/25F, PGTV50GY/25F. At the same time, 500 mg of apatinib was given every day. On August 29, 2019, intensity-modulated radiotherapy for chest tumors was started. The specific dose was PTV60GY/30F. The abdomen and pelvis CT in October 2019 showed that the bone metastases (Figure 1, B1–2) were partially relieved, and the liver lesions (Figure 1, C1-2) were stable. After 4 months of apatinib treatment, the abdomen CT in December 2019 (Figure 3, D1-2) showed that the number of liver lesions was slightly greater than before. The AFP was 8.03 μg/l. Considering that the patient had many liver lesions, it was recommended that the patient receive local radiotherapy combined with apatinib targeted therapy. Therefore, liver radiotherapy was applied from December 2, 2019 to January 7, 2020, and the specific dose was PTV48GY/24F. During this period, the patient’s general condition was normal and no serious adverse reactions occurred. The abdomen CT showed that the change of liver lesions was not obvious, and the AFP was 12.29 μg/l (Figure 4). According to relevant information, the combination of antiangiogenic drugs and immune checkpoint inhibitors can promote the normalization of blood vessels, reverse the immunosuppressive state, and enhance the anti-tumor immunity [3]. The synergistic effect of both drugs has also been verified in the KEYNOTE-524 study [4]. Therefore, after the MDT discussion, it was recommended to add a PD-1 inhibitor in order to enhance the curative effect and achieve greater relief in the patient’s treatment evaluation. Because the binding surface of the Fab segment of tislelizumab and PD-1 overlaps with the binding surface of PD-1/PD-L1 by as much as 82%, it can more completely block the binding of PD-1/PD-L1. Therefore, the patient was started on 200 mg of tislelizumab every 3 weeks combined with 500 mg of apatinib every day in June 2020. In November 2020, the abdomen CT showed that the liver lesions were stable, and the AFP was 60.40 μg/l, which was slightly higher than before. In March 2021, the abdomen CT (Figure 3, E1-2) showed that some lesions were enlarged. The AFP was 293.10 μg/l, which was significantly higher than before. We determined that the disease was progressive. Considering the possibility of drug resistance after more than 17 months of apatinib treatment, we suggested that the patient take 8 mg of lenvatinib every day combined with 200 mg of tislelizumab every 3 weeks from April 2021 to the present time. On August 6, 2021, the abdomen CT (Figure 3, F1-2) showed that the liver lesions were slightly larger than before, and the intrahepatic lesions were arterial and significantly enhanced unevenly. The AFP was 1086 μg/l, which was significantly higher than before. Considering the short time of use of lenvatinib, after consultation with the patient, TACE was performed on August 12, 2021, and 10 mg of lipiodol, 30 mg of lobaplatin, and 30 mg of pirarubicin were infused during the operation. The tumor index was re-examined on August 20, 2021 after surgery, and the AFP was 169.60 μg/l, which was significantly lower than that before surgery. On October 14, 2021, the abdomen CT showed that the liver lesions were stable.
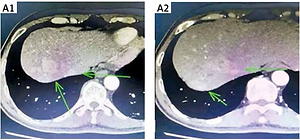
Figure 1
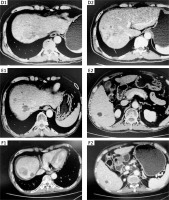
Patient computed tomography (CT) plain scan + enhanced: A1–2 – liver lesions in July 2019; A3 – parasternal lesion in July 2019; A4 – lumbar paravertebral lesion in July 2019. B1–2 – the bone metastases in October 2019; C1–2 – the liver lesions in October 2019
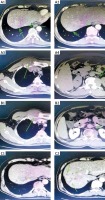
Figure 2
A – HCC under H + E staining at 100× microscope. B – HCC under H + E staining at 200× microscope
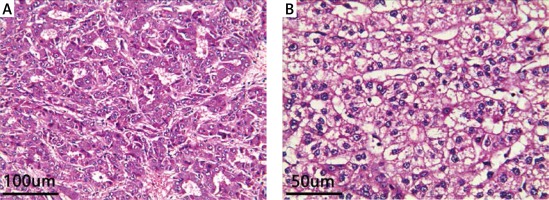
Figure 3
Patient computed tomography (CT) plain scan + enhanced. D1–2 – liver lesions in December 2019; E1–2 – liver lesions in March 2021; F1–2 – liver lesions on August 6, 2021
Discussion and Conclusion: HCC has a dynamic development process, and it is still a major problem and challenge for China and other countries. The CSCO guidelines for the diagnosis and treatment of primary liver cancer are formulated in light of China’s national conditions and specific circumstances. Patients with IIIA/IIIB liver cancer should be treated with local combined systemic therapy, including targeted maintenance therapy, TACE, radiotherapy, and surgical resection. Reviewing the occurrence and development of the patient’s disease, the patient had multiple intrahepatic tumors with bone metastasis at the onset, and was diagnosed with advanced HCC. Median OS varied significantly based on performance status (grade 1: 38.6 months, grade 2: 22.3 months) and presence of extrahepatic spread (11.2 months) or macrovascular invasion (MVI; peripheral portal vein thrombosis 11.2 months, main portal vein thrombosis 7.1 months) [5]. In the present case, the patient had extrahepatic metastases and tumor thrombus formation in the portal vein. The survival time was greater than 36 months, and the ECOG score of the patient was still 1 point.
In advanced HCC, tumor angiogenesis plays a key role in growth and development through VEGF and VEGF receptors. Apatinib, researched by the Chinese company Hengrui, is a new tyrosine kinase inhibitor of VEGFR-2 targeting the ATP binding site of receptor cells. It can inhibit the migration and proliferation of endothelial cells stimulated by VEGF, reduce tumor microvascular density, and promote apoptosis [6, 7]. In December 2020, it was approved for liver cancer indications in China. Web et al. [8] also reported that apatinib is effective as a first-line treatment for patients with advanced HCC. In their study, patients with advanced HCC were randomized to two groups, apatinib 850 or 750 mg daily until disease progression. The time to progression (TTP) in the 850 mg and 750 mg groups was 4.21 and 3.32 months, respectively (p > 0.05). In our case, the patient had been on apatinib for 17 months. After taking apatinib for 6 months, the efficacy was evaluated by partial remission. To increase the efficacy, combined treatment with tislelizumab was continued for more than 9 months. After re-examination, unfortunately, the patient’s liver lesions progressed more than before. Combined with the patient’s condition and previous experience, we were more inclined to the development of apatinib. Thus, we switched to lenvatinib. Lenvatinib is an oral small-molecule inhibitor of multi-receptor tyrosine kinase developed by Eisai, which can inhibit VEGFR 1–3, fibroblast growth factor receptor 1–4, and PDGF receptors (Body α, KIT and RET) [9], with more targets than apatinib. A phase 2 study showed favorable response to treatment in advanced HCC patients with acceptable toxicity, and an objective response rate (ORR) of 37% as assessed by Modified Response Evaluation Criteria in Solid Tumors compared with that of RECIST version 1.1 (24% ORR). The TTP was 7.4 months, and the median OS was 18.7 months [10]. According to the American Association for the Study of Liver Diseases guidelines in 2018, lenvatinib is recommended for patients with advanced intermediate BCLC after TACE progression [11]. So far, lenvatinib has been used for more than 5 months. We know that targeted therapy for advanced HCC is still under investigation.
PD-1, an immune checkpoint receptor expressed on T lymphocytes, is overexpressed in many types of human tumors in order to help escape the host immune system via PD-1/PD-L1 signaling. Tislelizumab, an investigational humanized IgG4 monoclonal antibody, is an anti-tumor immune drug developed by BeiGene, China, and has been studied in hematological cancers and advanced solid tumors. It was approved in China in December 2019 for patients with relapsed or refractory classical Hodgkin lymphoma after at least second-line chemotherapy [12]. The underlying mechanism of its therapeutic resistance is its high affinity and binding specificity for PD-1, which is designed to minimize binding to macrophage FcγR to eliminate antibody-dependent phagocytosis [13]. However, anti-PD-1 mAb non-specifically activates T cells, and immunity can mediate tissue damage or immune-related adverse reactions. The most common adverse reactions are pneumonia, hepatitis, hypophysitis, and rash, but these are usually reversible [14]. In the present case, fortunately, the patient did not have any immune-related adverse reactions.
TACE is currently one of the local treatments for HCC with compensatory ability. After TACE treatment, about 50% of patients’ tumors become smaller, and patients survive for 2 years. The rate reached 55.4% [15, 16]. According to the research results obtained by the team led by Pinato from Imperial College London, from the perspective of the immune microenvironment, TACE treatment induced CD8+/PD-1+ cells in liver cancer and decreased Treg cells, while promoting immune and inflammatory responses in the microenvironment; however, after TACE treatment, there was no difference in the expression of co-inhibitory proteins including PD-L1, IDO-1, Lag-3, Tim-3, or CTLA-4 [17]. This also shows that TACE has a certain curative effect on advanced liver cancer, but it still has limitations. The changes in the immune microenvironment revealed by TACE can provide new research directions for the exploration of new immunotherapy and related molecular targeting.
Local treatment combined with systemic treatment is recommended for advanced HCC. Although there are a variety of targeted and immune drugs, the effects of various drugs vary from person to person, which requires our clinicians to adopt individualized therapy. In the present case, through a comprehensive treatment plan, local radiotherapy, TACE, and immunotherapy combined with targeted therapy, the efficacy and quality of life were far superior to those of a single mode of treatment. Moreover, the survival period was significantly prolonged. We hope that there will be more evidence to demonstrate the efficacy and safety of combined treatment of HCC.