Introduction
A prandial and basal insulin (insulin detemir (IDet) or insulin glargine (IGlar)) have not been co-formulated so far because of the pharmacodynamic properties precluding their integration [1]. The development of insulin degludec (IDeg), a basal insulin that forms stable dihexamers in pH-6 physiological solution, has provided the possibility of co-formulation with another insulin analogues [2]. Insulin degludec/aspart (IDegAsp) is the first soluble combination that provides separate basal and prandial effects without requiring resuspension [3]. Insulin degludec/aspart is an IDeg and insulin aspart (IAsp) formulation that dissolves at a rate of 70 : 30 [2]. Insulin degludec (IDeg; Des (B30) LysB29 human insulin) is an ultra-long acting insulin analogue with a duration of effect estimated to be approximately 42 h, which is much longer than IGlar U100 and IDet [4, 5]. In the pharmaceutical preparation, the IDeg component forms dihexamers that are soluble at neutral pH, whereas the IAsp component remains as separate monohexamers. Following injection, IDeg di-hexamers rapidly form stable multi-hexamers from which IDeg monomers slowly and continuously dissolve in subcutaneous tissue. However, IAsp hexamers readily separate into monomers, which enable rapid absorption into the bloodstream. This stable pharmacokinetic profile may give rise to low fluctuations in glucose levels [6]. At the beginning of insulin therapy or after any dose change, the degludec component of IDegAsp reaches steady state plasma levels within two to three days [7]. Insulin degludec/aspart does not accumulate in plasma because its elimination rate is equal to the 24-hour absorption rate [4]. Insulin degludec/aspart is associated with lower risk of hypoglycaemia and more stable fasting plasma glucose control compared to premixed insulin [8, 9]. Because the basal-bolus insulin regimen is complex and requires a large number of daily injections, the combination of basal and prandial insulin can potentially reduce an important burden on patients by reducing the number of injections. Insulin degludec/aspart is a double-acting insulin that provides both prandial and basal glycaemic coverage without the need for multiple injections [10–13].
Diabetes presents a problem of complexity with high mortality and morbidity. Early studies have demonstrated the importance of providing optimal glycaemic control in reducing all-cause mortality in patients with diabetes [14]. Knapnik et al. evaluated the intensive care unit admissions and mortality in the Polish population. They reported that 24.7% of the ventilated and 22.2% of the non-ventilated patients had diabetes [15]. The poor prognosis seen with suboptimal glycaemic control provides an argument to study the potential benefits of new therapies in diabetes. Insulin degludec/aspart has been available for approximately 2 years in Turkey. The aim of this study was to demonstrate the efficacy and safety of transition from premixed and intensive insulin therapies to IDegAsp therapy.
Material and methods
Study design
The clinical trial protocol was approved by the Ethics Committee of Adıyaman University Education and Research Hospital (Date: 26/06/2019 Number: 2019/5-13) and complied with the Declaration of Helsinki. This retrospective cohort study was carried out between November 2017 and November 2018 in the Department of Endocrinology and Metabolism Diseases of Adıyaman University Education and Research Hospital. In this 1-year period, all patients who met the inclusion criteria were included in the study in a consecutive manner after providing written, informed consent.
Eligibility
Inclusion criteria: 1) male and female patients aged 18 years and over; 2) documented type 2 diabetes mellitus (type 2 DM); 3) patients on premixed or intensive insulin therapy in addition to oral antidiabetic drugs.
Exclusion criteria: 1) patients with acute coronary syndrome, cerebrovascular event, pregnancy, heart failure, chronic liver disease, renal function test abnormality, and cancer; 2) patients on treatments that can impair glucose metabolism, such as glucagon-like receptor agonist, anti-obesity drugs, and systemic or local steroid therapy; 3) patients with known or suspected alcohol addiction and using narcotic or illegal drugs.
Treatment and follow-up
Group 1
This group was composed of 55 patients who had diagnosed type 2 DM for at least eight years with glycated haemoglobin (HbA1c) levels between 7.3 and 14.4% and body mass index (BMI = body mass [kg]/height [m2]) of ≤ 40 kg/m2. Participants were on twice-daily (morning and evening) premixed (biphasic insulin aspart 30 (BIAsp 30) or biphasic insulin lispro mix 25) insulin for at least 3 months ahead of switching to IDegAsp. At the time of transition to IDegAsp and on the 12th week of treatment, fasting plasma glucose (FPG), morning postprandial plasma glucose (PPG), HbA1c level, body mass, BMI, daily total insulin doses, and confirmed symptomatic hypoglycaemia episodes (self-reported plasma glucose < 56 mg/dl or 3.1 mmol/l) of patients were recorded.
Group 2
This group included 60 patients with type 2 DM for at least 3 years, HbA1c levels between 6.6 and 13.9%, and BMI ≤ 40 kg/m2. Participants were on one kind of bolus prandial insulin (glulisine, aspart, or lispro) three times a day and a daily single dose of IGlar or IDet as basal insulin treatment for at least 3 months prior to switching to IDegAsp. At the time of transition to IDegAsp and on the 12th week of treatment; FPG, PPG, HbA1c level, body mass, BMI, daily total insulin doses, and confirmed symptomatic hypoglycaemia episodes (self-reported plasma glucose < 56 mg/dl or 3.1 mmol/l) of patients were collected.
Patients in both groups continued their strict diet regimen and exercise programs. The oral antidiabetic drugs (data on oral antihyperglycaemic treatments are presented in Table I) being used were not changed, and their dosage was not titrated during the transition period or during the 12-week follow-up time. The switch from current insulin therapy to twice-daily IDegAsp co-formulation was performed according to the results of intensive blood glucose measurements (eight times a day) and hypoglycaemic events experienced in the last week [16].
Table I
Data of oral antidiabetic drugs used by patients in group 1 and group 2
Biochemical analyses
Fasting blood samples of all patients were taken from the antecubital vein after fasting overnight (at least 8 h). Postprandial blood samples were taken 2 h after the start of breakfast. Biochemical parameters were studied from plasma samples. Plasma glucose levels were measured by enzymatic reference method with hexokinase (Cobas c 501, Mannheim, Germany), and plasma HbA1c levels were measured by high-performance liquid chromatography and mass spectroscopy method (Adams A1C HA-8160, Koka, Japan).
Statistical analysis
Normality of distribution was examined by using both the Kolmogorov-Smirnov and Shapiro-Wilk W test. The results of Shapiro-Wilk test were chosen because it is more powerful in estimating departures from normality in small samples. Descriptive statistical methods including percentage and mean ± standard deviation (± SD) or median (interquartile range (IQR)) were used to provide the basic features of the data, according to the evaluation of distribution for normality. Paired samples t test was used for normally distributed continuous variables (body mass, BMI, duration of insulin use, HbA1c, FPG, PPG, confirmed symptomatic hypoglycaemia) for group 1 and group 2. Wilcoxon signed-rank test was used for non-normally distributed continuous variables (duration of diabetes, total daily insulin dose) for group 1 and group 2. The comparisons of the data of the two groups were performed using the Mann-Whitney U test (for comparison of total daily insulin dose) and independent sample t test (for comparison of body mass, BMI, confirmed symptomatic hypoglycaemia, FPG, PPG, and HbA1c). All the patients who met the inclusion criteria were retrieved from our institution database. To determine the sample size, power analysis was carried out with the use of G*Power (v3.1.9) program. Predicting a large effect size (d = 0.8) would be detected, a minimum of 26 patients per group was calculated to be required to provide a power of 80% at a significance level of α = 0.05. All statistical analyses were carried out using SPSS 23.0 (IBM Corporation, Armonk, NY, US). Two-tailed p < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
A total of 115 patients were included in the study. The characteristics of the participants including age, sex, HbA1c, duration of diabetes, body mass, and BMI were well balanced between the two treatment groups. Of the 55 patients in group 1, 29 were male and 26 were female. The median age was 67.0 (62.0–69.0) years, duration of diabetes was 15.0 (10.0–18.0) years, and mean duration of insulin use was 7.9 ±2.9 years. Of the 60 patients in group 2, 32 were male and 28 were female. The median age was 61.5 (54.7–65.7) years, duration of diabetes was 14.0 (10.0–20.0) years, and mean duration of insulin use was 7.5 ±2.84 years (Tables II and III).
Table II
Pre- and post-switch differences in Group 1 patients
| Characteristic | Premixed treatment | IDegAsp treatment |
|---|---|---|
| Age [years]* | 67.0 (62.0–69.0) | |
| Sex male/female, n (%) | 29/26 (53/47) | |
| Body mass [kg]† | 83.3 ±8.1 | 81.8 ±8.0 |
| BMI [kg/m2]† | 29.8 ±3.9 | 29.2 ±3.5 |
| Duration of diabetes [years]* | 15.0 (10–18) | 15.0 (10–18) |
| Duration of using insulin [years]† | 7.9 ±2.9 | 7.9 ±2.9 |
| HbA1c (%)† | 10.6 ±1.9 | 8.7 ±1.6 |
| FPG [mmol/l; mg/dl]† | 13.2 ±4.2; 238.7 ±75.9 | 9.2 ±3.1; 165.8 ±56.9 |
| PPG [mmol/l; mg/dl]† | 19.4 ±4.2; 351.1±76.6 | 14.7 ±3.8; 266.3 ±69 |
| Insulin dose [U/day; U/kg/day]* | 48 (40–55); 0.56 (0.49–0.64) | 38 (36–40); 0.46 (0.40–0.50) |
| Confirmed symptomatic hypoglycaemia (event/week)† | 1.5 ±0.85 | 0.03 ±0.11 |
| Pre-study antidiabetic regimen: | ||
| Biphasic insulin aspart 30/70 | 42 | 55 |
| Biphasic insulin lispro 25/75 | 13 | – |
Table III
Pre- and post-switch differences in group 2 patients
| Characteristic | Intensive treatment | IDegAsp treatment |
|---|---|---|
| Age [years]* | 61.5 (54.7–65.7) | |
| Sex male/female, n (%) | 32/28 (53/47) | |
| Body mass [kg] (SD)† | 83.9 ±12.8 | 82.9 ±12.8 |
| BMI [kg/m2] (SD)† | 31.2 ±3.7 | 30.9 ±3.8 |
| Duration of diabetes [years]* | 14 (10–20) | 14 (10–20) |
| Duration of using insulin [years]† | 7.5 ±2.8 | 7.5 ±2.8 |
| HbA1c (%)† | 10.5 ±1.98 | 8.9 ±2.0 |
| FPG [mmol/l; mg/dl] (SD)† | 11.7 ±3.52; 211.3 ±63.6 | 10.1 ±4.11; 182.4 ±74.2 |
| PPG [mmol/l; mg/dl] (SD)† | 18.2 ±5.11; 328.1 ±92.1 | 13.4 ±3.85; 242.6 ±69.4 |
| Insulin dose [U/day; U/kg/day]* | 67 (60–85.2); 0.85 (0.65–1.0) | 40 (36–48); 0.50 (0.40–0.58) |
| Confirmed symptomatic hypoglycaemia (events/week)† | 0.93 ±1.17 | 0.07 ±0.25 |
| Pre-study antidiabetic regimen: | ||
| Insulin aspart + glargine | 20 | – |
| Insulin aspart + detemir | 16 | 60 |
| Insulin lispro + glargine | 12 | – |
| Insulin glulisine + glargine | 12 | – |
Glycaemic control
After transition to IDegAsp, HbA1c values decreased significantly in both groups. In group 1, the mean HbA1c was 10.6 ±1.9% before the transition and 8.7 ±1.6% after the treatment with IDegAsp (p < 0.0001). The mean HbA1c was 10.5 ±1.9% before the transition in group 2 and 8.9 ±2.0% after the treatment with IDegAsp (p < 0.0001).
Significant FPG reductions were observed after transition to IDegAsp treatment. In group 1, the mean FPG before treatment was 238.7 ±75.9 mg/dl, whereas the mean FPG obtained after 12 weeks was 165.8 ±56.9 mg/dl (p < 0.0019). In group 2, the mean FPG was 211.3 ±63.6 mg/dl and 182.4 ±74.2 mg/dl before and after the 12-week treatment of IDegAsp, respectively (p = 0.007).
The mean PPG of group 1 before the transition was 351.1 ±75.9 mg/dl, whereas the mean PPG obtained after 12-week treatment of IDegAsp was 266.3 ±69.0 mg/dl (p < 0.0001). Similarly, the mean PPG of group 2 before the treatment was 328.1 ±92.1 mg/dl, whereas it was 242.6 ±69.4 mg/dl on the 12th week of IDegAsp (p < 0.0001).
Insulin dose
The median daily insulin dose in group 1 was 48 (40–55) U/day while on premixed insulin, which was decreased significantly to 38 (36–40) U/day by using IDegAsp after 12 weeks of treatment (p < 0.001). And the dose of insulin decreased from 0.56 (0.49–0.64) U/kg to 0.46 (0.40–0.50) U/kg daily with 12-week treatment of IDegAsp (p < 0.001).
In group 2, the median total dose of insulin was 67 (60–85.2) U/day while on intensive insulin, whereas it was 40 (36–48) U/day after 12 weeks on IDegAsp treatment (p < 0.0001). And the dose of insulin decreased from 0.85 (0.65–1.0) U/kg to 0.50 (0.40–0.58) U/kg daily with 12-week treatment of IDegAsp (p < 0.0001).
Body mass and mass index
In group 1, the mean body mass was 83.3 ±8.1 kg and 81.8 ±8.0 kg before and after the treatment with IDegAsp, respectively (p = 0.011). The mean BMI was 29.8 ±3.9 kg/m2 before treatment change and 29.2 ±3.5 kg/m2 at the end of 12 weeks of IDegAsp (p = 0.012). Similar to group 1, the estimated mean body mass in group 2 was 83.9 ±12.89 kg and 82.9 ±12.8 kg before and after the treatment change, respectively (p = 0.005). The mean BMI was 31.2 ±3.7 kg/m2 before IDegAsp treatment and 30.9 ±3.8 kg/m2 afterwards (p = 0.012).
Hypoglycaemic events
The rate of hypoglycaemic events decreased significantly on IDegAsp treatment. Episodes of confirmed hypoglycaemia were 1.5 ±0.85/week before the treatment switch in group 1 which decreased to 0.03 ±0.11/week after IDegAsp use (p < 0.0001). With regard to group 2, while the episodes of confirmed hypoglycaemia were 0.93 ±1.17 per week before the treatment transition, hypoglycaemic events decreased to 0.07 ±0.25/week after the institution of IDegAsp (p < 0.0001).
Comparison of group 1 and group 2
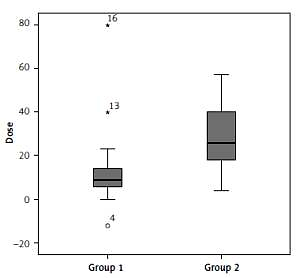
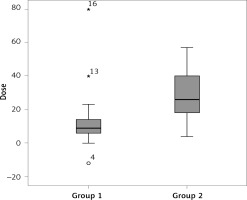
There was no significant difference across the groups regarding the pre- and post-IDegAsp change in HbA1c, FPG, PPG, body mass, BMI, and hypoglycaemic events, except the median total daily insulin dose reduction – group 1 had relatively few compared to group 2 (p = 0.001) (Table IV and Figure 1).
Table IV
Comparison of changes in parameters after insulin degludec/aspart treatment
| Compared parameters | Group 1 | Group 2 | P-value |
|---|---|---|---|
| Δ Insulin dose: | |||
| U/day* | –9 (6–14.5) | –26 (17.5–40) | 0.001 |
| U/kg/day* | –0.10 (0.07–0.16) | –0.31(0.21–0.46) | 0.001 |
| Δ Body mass† | –1.45 ±2.80 | –1.00 ±1.81 | 0.482 |
| Δ BMI† | –0.55 ±1.13 | –0.34 ±0.65 | 0.400 |
| Δ Hypoglycaemia† | –1.45 ±0.81 | –0.86 ±1.25 | 0.056 |
| Δ FPG† | –72.9 ±79.3 | –28.8 ±86.2 | 0.066 |
| Δ PPG† | –84.8 ±86.6 | –85.4 ±102.6 | 0.981 |
| Δ HbA1c† | –1.90 ±1.96 | –1.62 ±2.09 | 0.635 |
Discussion
This 12-week, real-world evidence, combined analysis revealed significant improvements in glycaemic control, weight loss, and hypoglycaemic events with switch to IDegAsp co-formulation from both premixed and intensive insulin therapies in patients with type 2 DM. Furthermore, a significant decrease was encountered in the median total daily insulin dose.
Use of premixed and intensive insulin is quite common in daily practice in the population of diabetic persons in many countries; hence, both patients and physicians are highly experienced in the administration and more importantly regarding the impact and side effects of the regimen. The American Diabetes Association specifies that longer-acting basal analogues (degludec) may convey a lower hypoglycaemia risk compared to basal insulin treatment when used in combination with oral agents [17]. With the introduction of IDegAsp co-formulation, in comparison to premixed insulins, more advantages such as reduction in the number of hypoglycaemic events have been provided [9, 18]. Furthermore, the IDegAsp co-formulation has been shown to result in less complexity in patients’ daily lives and potentially improves glycaemic control when compared with concentrated insulin therapy in the basal-bolus regimen [19]. Existing studies comparing twice-daily IDegAsp with BIAsp 30 treatment showed that IDegAsp treatment was non-inferior to BIAsp treatment with regard to HbA1c, whereas the reduction in FPG with IDegAsp was higher than that with BIAsp 30 treatment [20, 21]. Inconsistent with randomised controlled trials, our retrospective study, which included real-world data, reported significant HbA1c decline with IDegAsp by transition from both biphasic and intensive insulin regimens. In a study by Liebl et al. in an Asian population, IDegAsp treatment, when compared to BIAsp 30, resulted in a higher reduction in mean daily total insulin dose [20]. In our study, the insulin dose reduction of the patients in group 1 was found to be consistent with previous reports; however, the most dramatic result was that even after switching from a strong, effective, and ideal insulin treatment such as basal bolus regimen, IDegAsp came up with a decreased insulin need, as well. A statistically significant advantage in FPG, PPG, and HbA1c along with daily total insulin dose gained from IDegAsp in our population supported this regimen as a potent intervention in glycaemic control. Some physicians prefer not to use fixed-dose insulin preparations because of the belief that separation of basal and bolus components allows better adaptation of insulin dosages to patients’ needs [22]. In a 12-week study comparing once-daily IDegAsp and basal insulin groups, HbA1c change, daily insulin dose, and overall frequency of hypoglycaemia did not differ between the two groups [23]. Furthermore, Kawaguchi et al., in their recently published study using the flash glucose monitoring system, demonstrated that the IGlar U300/insulin glulisine (basal-bolus treatment) was superior to IDegAsp in terms of efficacy and safety [24]. Our findings can be viewed as contradicting the previously held idea on the superiority of basal-bolus treatment over fixed-dose insulin preparations.
Any benefit from hypoglycaemic agents must be weighed against the adverse events. The occurrence of hypoglycaemia episodes is a limiting factor to provide adequate metabolic control in insulin-treated DM patients, particularly for those with unstable diabetes [25]. The risk of hypoglycaemia is directly related to increased glycaemic variability [26–28]. Therefore, one of the purposes is to reduce the fluctuations in glucose levels during the evaluation and development of new treatments [29]. Not unexpectedly, the occurrence of hypoglycaemia (confirmed, nocturnal, and severe) is lower with IDegAsp than with BIAsp 30 treatment [30]. In a meta-analysis consisting of seven clinical trials, IDeg was shown to be associated with equivalent HbA1c control with IGlar and a significantly lower rate of nocturnal hypoglycaemia as compared to it [31]. Although the nocturnal hypoglycaemia rates were not recorded in the present study, one of the most striking results of our own data is the significant reduction in the rate of hypoglycaemic episodes.
The frequency of hypoglycaemia in response to changing insulin therapy may vary significantly among patients [2, 32]. Although it is recommended that the dose remain unchanged during the transition from premixed or intensive injection regimen to the IDegAsp, it is still advisable to determine the doses according to the patient’s specific needs in all switches. The results of intensive blood glucose measurements and the rate of hypoglycaemic events in the week prior to switch from current insulin therapy to IDegAsp co-formulation are the most important mediators of determining a patient-specific insulin dose. As such, in the present study, due to the high frequency of hypoglycaemic events in group 1 and group 2, the switch to IDegAsp could not be performed at the recommended 1 : 1 doses [16].
Selection bias resulting from retrospective work is our main limitation. Second is the absence of records of patients other than the baseline and 12th week estimates. The third limitation is the small sample size and lack of a control group. Another limitation relates to the short course of the treatment. Additional follow-up is needed before it can be concluded that the optimal glucose levels were achieved because the glycaemic control remained relatively weak in both groups at the end of the study. Also, a 12-week duration is not adequate to document the changes in body mass and BMI.
In conclusion, weight loss and less total insulin requirement coupled with reduced hypoglycaemia were detected after the transition from premixed and intensive treatment regimens to IDegAsp treatment. The switch to IDegAsp treatment is a reasonable option for clinical circumstances such as frequent hypoglycaemic episodes, use of complex multi-drug regimens, and when targeting low total insulin dose. Our study is strengthened by the paucity of a randomised controlled trial to date, comparing IDegAsp treatment with intensive insulin regimen, and it is particularly important for pointing out the difference from randomised trials, concerning the HbA1c outcome in a real-world clinical use of insulin treatment. Although long-term studies targeting the outcomes of IDegAsp treatment are needed, it has the potential to benefit in a variety of patient groups with type 2 DM.