Atrial fibrillation (AF) is one of the most prevalent cardiac arrhythmias, and primary hypertension is a common risk factor and a frequent comorbidity of AF [1]. Presence of hypertension in AF patients substantially increases the risks of developing heart failure and stroke [1]. Thus, clarifying the factor(s) linking AF and primary hypertension may benefit the treatment of AF-hypertension comorbidity. Evidence has implicated immune cell dysfunction as a significant contributor in the development of both AF [2–6] and hypertension [7–10].
Immune-mediated inflammation is increasingly recognized as a key driver of AF pathogenesis [3]. Studies have identified increased infiltration of T cells in the left atria of AF patients, and the degree of infiltration was positively correlated with atrial enlargement [4, 5]. Additionally, AF patients exhibit dysregulation in circulating CD4+ T cell subsets [6].
Lymphocytes also play a pivotal role in hypertension development. A landmark study by Guzik et al. [8] first reported that recombination-activating gene 1-knockout (Rag1–/–) mice, which lack T and B lymphocytes, exhibit resistance to angiotensin II (Ang II)- and deoxycorticosterone acetate-induced hypertension. This finding was later confirmed by alternative studies using different mouse models [9, 10]. Further investigations revealed that specific T cell subsets, including pro-inflammatory T helper 17 (Th17) and CD8+ T cells, promote hypertension, whereas regulatory T (Treg) cells exert protective effects.
Despite these advances, the characteristics of circulating lymphocytes and CD4+ T cell subpopulations in AF patients with primary hypertension remain elusive. Here, we comprehensively analyzed the absolute counts and relative percentages of circulating lymphocytes and CD4+ T subpopulations in AF patients with or without primary hypertension, aiming to determine the independent immune-related factors for AF-hypertension comorbidity. Our findings may provide novel insights for improving the therapeutic strategies and prognostic assessments in clinical management of the comorbidity.
Methods
Patients with a diagnosis of AF with primary hypertension (n = 15) or AF with normal blood pressure (n = 15) were enrolled in Shanxi Cardiovascular Hospital within the period April 2023 to October 2023. All participants underwent radiofrequency ablation procedures. Both AF and hypertension were diagnosed based on the guidelines issued by the American College of Cardiology/American Heart Association and the European Society of Cardiology. The exclusion criteria comprised valvular disease, congenital heart disease, cardiomyopathy, heart failure, autoimmune disease, acute infection, hepatic and renal dysfunction, malignancy, and recent use of anti-inflammatory or immunosuppressive medications. The study was approved by the Research Ethics Committee of Shanxi Cardiovascular Hospital (approval ID 2023xxg001). Written informed consent was obtained from all patients.
Venous blood samples were collected from patients on the morning of the radiofrequency ablation day after an overnight fast and before the administration of any procedural medications and the ablation procedure. The BD FACSCalibur platform (BD Biosciences) was used to detect the following blood lymphocytes: T (CD45+CD3+), CD4+ T (CD45+CD3+CD4+), CD8+ T (CD45+CD3+CD8+), B (CD45+CD3-CD19+), natural killer (NK) (CD45+CD3-CD16+CD56+), T helper 1 (Th1) (CD4+ IFN-γ+), T helper 2 (Th2) (CD4+ IL-4+), Th17 (CD4+ IL-17+), and Treg (CD4+IL-25+CD127low) cells. Multitest antibody panel A (CD3-fluorescein isothiocyanate (FITC)/CD8-allophycocyanin (APC)/CD45-peridinin chlorophyll protein (PerCP) / CD4- phycoerythrin (PE)) and panel B (CD3-FITC/CD16+56-PE/CD45-Percp/CD19-APC) (BD Biosciences) were used to detect circulating lymphocytes A (T, CD4+ T, and CD8+ T cells) and B (B and NK cells), respectively. The MultiSET software was used to determine the percentages and absolute counts of circulating lymphocytes.
To detect Th1, Th2, Th17, and Treg cells, CD4-FITC, IL-4-PE, IFN-γ-APC, IL-17-PE, CD25-APC, and CD127-PE (BD Biosciences) were used. The CellQuest software was used to determine the percentages of CD4+ T subsets. The absolute numbers of cells were calculated by the percentage of each subpopulation multiplied by the absolute count of CD4+ T cells.
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and were compared using Student’s t-test. Categorical variables, expressed as numbers (percentages), were compared using the χ2 test. Correlations between CD4+ T cell subsets and blood pressure levels were evaluated using Pearson correlation analysis. The independent association between CD4+ T cell subsets and hypertension was examined using logistic regression analysis. The statistical analyses were performed with IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). The forest plot was carried out using R software (version 4.4.3). Two-tailed p- values of < 0.05 were considered statistically significant.
Results
Age, sex, prevalence of coronary artery disease (CAD), diabetes mellitus (DM), stroke, and paroxysmal AF, use of NOAC (new oral anticoagulants), and statin did not significantly differ between the hypertensive and normotensive AF patients (Table I). As expected, both systolic blood pressure (SBP) (139.7 ±16.7 vs. 118.8 ±12.8 mm Hg) (p = 0.001) and diastolic blood pressure (DBP) (82.3 ±9.4 vs. 75.0 ±8.1 mm Hg) (p = 0.030) were significantly higher in the hypertensive group compared to the normotensive controls.
Table I
Patients’ characteristics
Flow cytometric analysis revealed no significant difference either in the absolute counts or in the relative percentages of major lymphocyte populations (T cells, B cells, NK cells, CD4+ T cells, and CD8+ T cells) between hypertensive AF patients and normotensive AF patients. However, detailed examination of CD4+ T cell subsets (Th1, Th2, Th17, and Treg cells) demonstrated that the hypertensive AF group exhibited reduced Treg cell percentages (3.3 ±0.6% vs. 4.2 ±0.9%) (p = 0.003) and elevated Th17/Treg ratio (0.4 ±0.1 vs. 0.3 ±0.1) (p = 0.004) compared to the normotensive AF group.
Pearson correlation analysis revealed that SBP was negatively correlated with the percentage of Treg cells and positively correlated with Th17/Treg ratio, whereas DBP was positively correlated with the percentage of Th1 cells and Th1/Th2 ratio (Figure 1). The levels of Th2 and Th17 cells did not significantly correlate with SBP or DBP (Figure 1).
Figure 1
Pearson correlations between systolic blood pressure (SBP), diastolic blood pressure (DBP), and CD4+ T cell subsets. A – Correlation between SBP and CD4+ T cell subsets. B – Correlation between DBP and CD4+ T cell Subsets
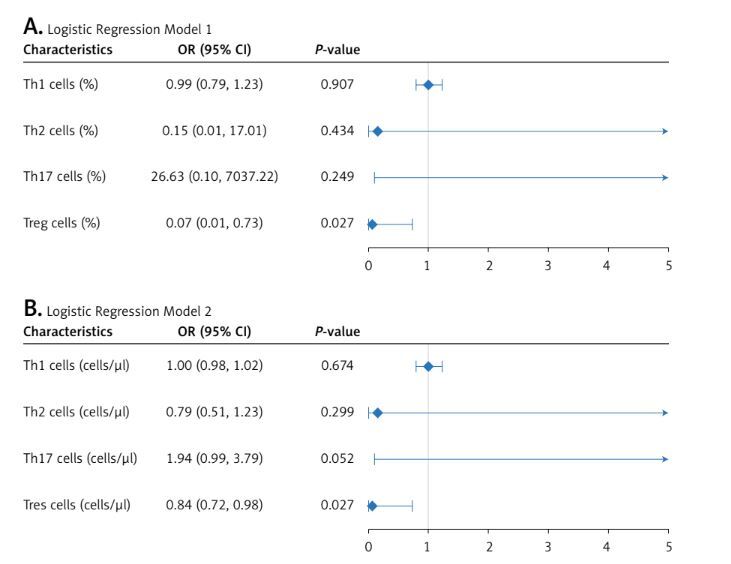
Multivariate logistic analysis was performed to exclude the effects of age, sex, and other CD4+ T cell subsets on hypertension in AF patients. Both the percentage and the count of Treg cells were associated with hypertension after adjusting for age, sex, and the levels of Th1, Th2, and Th17 cells (Figure 2). The results indicated that the role of Treg cells in the hypertension of AF patients was independent of other CD4+ T subsets.
Discussion
Here we systematically evaluated the relationship between circulating lymphocyte subsets (including counts and percentages) and primary hypertension in AF patients. The key findings were that 1) Treg cell percentage was significantly lower in hypertensive AF patients compared to normotensive AF patients; 2) SBP showed a negative correlation with Treg levels, while DBP had a positive correlation with Th1 cells; 3) after adjusting for age, sex, and Th1/Th2/Th17 subsets, both the counts and the percentages of Treg cells remained independently associated with the hypertension in AF patients.
Treg cells are key suppressors of inflammation and immune responses. Daniel et al. [11] demonstrated that adoptive transfer of Treg cells can attenuate Ang II-induced hypertension and vascular injury. Masato et al. [12] reported a reduced splenic Treg proportion in spontaneously hypertensive rats. However, human studies have yielded conflicting results: no difference in Treg numbers was observed between patients with secondary hypertension and normotensive controls [13], whereas hypertensive children exhibited reduced Treg frequencies [14]. Here we demonstrated that AF patients with primary hypertension had fewer circulating Treg cells, reinforcing the potential role of Treg deficiency in primary hypertension.
Th17 cells are key producers of IL-17, and have been implicated in the pathogenesis of hypertension. Madhur et al. [15] first demonstrated that IL-17 is critical for the maintenance of Ang II-induced hypertension. Clinically, elevated Th17 cell numbers have been observed in patients with secondary hypertension [13]. However, here we did not find significant differences in Th17 cell levels between hypertensive and normotensive AF patients. This discrepancy may stem from the modest sample size of our study, which may potentially reduce the statistical power in evaluating the intergroup difference of the Th17 cell population. Th1 and Th2 cells have not been well characterized in hypertension. We observed here that DBP showed a positive correlation with Th1 cell percentage, suggesting that Th1 cells may have a specific role in DBP regulation, a function distinct from their effect on SBP.
CD8+ T cells are also main factors involved in hypertension. Trott et al. [16] found that CD8–/– mice are resistant to Ang II-induced hypertension, and adoptive transfer of CD8+ T cells restores hypertension in Rag1–/– mice. A subsequent study by Liu et al. [17] further showed that CD8+ T cells mediate Na-Cl cotransporter upregulation, and the development of salt-sensitive hypertension relies on direct cell-cell contact of CD8+ T cells with the distal convoluted tubule cells in kidneys. However, the present study did not reveal a significant difference in the circulating CD8+ T cell levels between hypertensive and normotensive AF patients. This null finding might either reflect a true lack of difference or be a result of limited statistical power. The phenomenon may alternatively suggest that the effects of CD8+ T cells on hypertension are predominantly mediated by the tissue-resident populations rather than by the circulating subsets. Although the involvement of T cells in hypertension has been extensively studied, the role of B and NK cells, particularly in patients with concurrent AF and hypertension, remains poorly evaluated. While CD8+ T cells and NK cells were detected in our analysis, their functional state (e.g., activation markers such as CD69 or granzyme B) and tissue-residency potential (e.g., CD103 for CD8+ T cells) remain to be explored. Clarifying these features could help elucidate their functional role in hypertension.
This study has several limitations. First, the sample size is relatively small, which may constrain the statistical power and potentially lead to false-negative results (Type II errors). Second, we did not perform functional experiments on the T cell subsets, including the Th cell-associated cytokine profiling and the suppressive activities of Treg cells. This shortcoming may restrict the mechanistic conclusions. Third, the lack of associated clinical endpoint data may limit the translational potential of the results. Future studies incorporating clinical endpoints are required to evaluate the potential causal relationships. Fourth, individual variations in perceived procedural stress may have influenced lymphocyte profiles, despite standardized sampling timing.
In conclusion, this study demonstrates that Treg cell level has an independent and negative association with primary hypertension in patients with AF. Meanwhile, Th1 cell level had a positive correlation with DBP but not SBP. There are no significant differences in CD8+ T cell, NK cell, B cell, Th2 cell, and Th17 cell levels between hypertensive and normotensive AF patients. These findings may advance our understanding on the functions of circulatory lymphocyte subpopulations in primary hypertension and hypertension-AF comorbidity.