Introduction
In developed western countries, aortic stenosis is the most common valvular heart disease in individuals over 65 years of age [1]. Surgical aortic valve replacement (SAVR) is the first-line therapy for symptomatic patients, improving prognosis and quality of life [2, 3].
Transcatheter aortic valve implantation (TAVI) may be performed among patients according to either a high surgical risk, a technical contraindication to surgery or a general poor condition named frailty [4, 5].
Frailty is a clinical syndrome combining decrease in physiological reserve and stress tolerance [6, 7] that can be assessed implicitly, while a comprehensive geriatric assessment (CGA) helps to globally assess medical and social issues of older adults based on a set of clinical scores, questionnaires and biological tests [8].
Heart team and geriatric evaluation units will assess every patient denied surgical therapy because of frailty to determine whether TAVI remains appropriate [9, 10].
The aim of the study was to identify whether CGA might be useful to improve the precision of the 1-year prognosis of presumably frail patients, whatever the management of aortic stenosis.
Material and methods
Study population
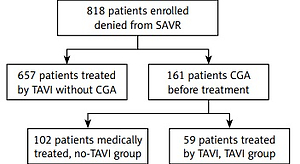
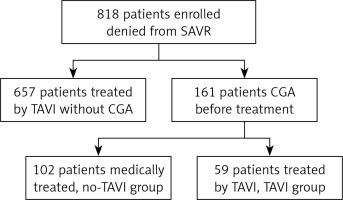
A total of eight hundred eighteen patients considered for TAVI were prospectively and consecutively enrolled between March 2011 and July 2016.
Inclusion criteria were a symptomatic severe aortic stenosis and being denied a SAVR by a heart team because of either a technical barrier (for instance porcelain aorta, chest deformation, or history of chest radiotherapy), a high preoperative risk as assessed by the EuroSCORE (a risk model for the prediction of mortality after heart surgery), or because of a heart team-assessed general poor condition to undergo thoracic surgery named frailty.
One hundred sixty-one patients presenting a general frail condition raised concerns about the direct individual benefits of undergoing TAVI; they necessitated a CGA before the final decision from the heart team and constituted the study population. The heart team included an interventional cardiologist, a non-interventional cardiologist, a heart surgeon and an anesthesiologist. The team took all CGA data into account to determine the most appropriate care.
The local ethical committee approved the research protocol (#2017/45) and the study was conducted in accordance with the Declaration of Helsinki and local regulatory requirements.
Data collection
All data related to demographic, morphometric, and echocardiographic parameters, and medical history were prospectively collected. Pulmonary hypertension was defined as a systolic pulmonary artery pressure of more than 35 mm Hg, moderate renal insufficiency by a creatinine clearance of less than 60 ml/min, respiratory insufficiency by the daily use of bronchodilator or inhaled corticosteroid, and history of pulmonary edema by at least two acute episodes during the last 12 months [11].
The CGA was performed by the geriatric evaluation unit during hospitalization to appreciate medical and social issues related to older adults. The main items were: an evaluation of polypharmacy based on the number of therapeutic classes used per day and the use of psychoactive drugs, memory by the learning and recall categories of the Mini-Mental State Examination (Short MMSE) [12] and the clock-drawing test (CDT) score [13], pain assessed by the verbal rating scale [14], nutrition by the short form of the Mini Nutritional Assessment (MNA) [15] and by the body mass index (BMI), independency by iso-resource group (GIR) [16] and Instrumental Activities of Daily Living (IADL) [17]), and mobility by the Five-Times-Sit-to-Stand Test (FTSST), which was considered as positive if accomplished without assistance and/or without use of upper extremity support [18].
Follow-up and outcomes
Follow-up was completed for all patients for at least 1 year with a median of 456 [153; 815] days. It involved immediate feedback provided by consultants and phone interview with a general practitioner. The primary endpoint was all-cause mortality at 1 year.
Statistical analysis
All statistical tests were performed using SPSS 20.0 software (IBM, Chicago, Illinois). Quantitative variables were expressed as mean ± standard deviations, unless stated otherwise, and qualitative variables as numbers and percentages. Comparisons of quantitative variables were conducted by means of unpaired Student’s t-test. Comparisons of qualitative variables were performed using the χ2 or Fisher’s test, as appropriate.
Cox regression models were applied to explain 1-year all-cause mortality. Multivariate analysis included common medical data, aortic stenosis parameters, and CGA results. Only significant univariate correlates (p < 0.05) were included into the multivariate Cox models. Specific interactions were tested between TAVI and NYHA score, FTSST, moderate renal failure and respiratory failure. Then, multivariate Cox regression models were performed separately on the TAVI and the no-TAVI groups.
Proportional-hazard assumptions were tested by analysis of the Schoenfeld residuals. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
Study population
CGA patients were older (85.9 ±4.6 vs. 82.8 ±7.1 years old, p < 0.001), with higher logistic EuroSCORE (29.5 ±17.5 vs. 19.5 ±12.6, p < 0.001), and lower LV ejection fraction (53.5 ±13.7 vs. 58.7 ±12.2%, p < 0.001) compared to patients who did not require CGA prior to TAVI. CGA patients presented more history of atrial fibrillation (51% vs. 32%, p < 0.001), but similar rates of history of diabetes, peripheral artery disease, stroke, renal failure, coronary artery disease, and symptoms related to aortic stenosis.
Out of 161 CGA patients, 102 patients did not undergo a TAVI (no-TAVI group) and 59 patients underwent a TAVI (TAVI group) (Figure 1). In the TAVI group, 14 patients had a CoreValve (Medtronic, Santa Ana, CA, USA) prosthesis and 45 had an Edwards Sapien, Sapien 3 or Sapien XT prosthesis (Edwards Lifesciences, Irvine, CA, USA). Forty-four (74%) patients had a transfemoral, 6 (10%) a transapical and 9 (16%) another approach. The TAVI was a success in 57 cases (96.6%) with no relevant procedural complications.
Mean age was 85.9 ±4.6 years (85.5 ±5.1 vs. 86.1 ±4.3; p=0.40) and mean BMI was 26 ±5 kg/cm2 (26.5 ±5 vs. 25.7 ±5.1; p = 0.36).
The no-TAVI group had a higher surgical risk score (logistic EuroSCORE 1: 33.4 ±17.8 vs. 22.7 ±14.9; p < 0.001) and higher rates of moderate renal insufficiency (82% vs. 57%; p = 0.001).
No-TAVI and TAVI patients presented similar rates of New York Heart Association (NYHA) functional class III or IV (71% in TAVI group vs. 56% in no-TAVI group; p = 0.06), history of pulmonary edema (35% vs. 36%; p = 0.88), and similar left ventricular ejection fraction and transvalvular mean pressure gradient. However, there was more pulmonary hypertension in the TAVI group (61% vs. 50%; p = 0.013) (Table I).
Table I
Patient characteristics at baseline, cardiovascular data
Geriatric characteristics
There was no difference regarding history of falls, number of therapeutic classes used per day, use of psychoactive drugs, social isolation and needs for home care worker support.
The no-TAVI group presented more memory impairment with a lower short MMSE and CDT score (respectively 36% of MMSE3 > 5 vs. 57%; p = 0.013 and 1.4 ±2.1 points vs. 2.7 ±2.4; p = 0.025). Moreover, the no-TAVI group was more dependent with a significantly lower GIR score than the TAVI group (3.6 ±0.9 vs. 4.2 ±0.9; p < 0.001) (Table II). FTSST was achieved in 18% of the cohort (respectively 15% vs. 25% for no-TAVI and TAVI group, p = 0.20).
Table II
Patient characteristics at baseline, geriatrics data
One-year mortality
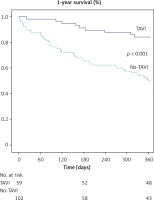
One-year mortality was 16% (n = 16) in the TAVI group and 46% (n = 27) in the no-TAVI group (p < 0.001) (Figure 2).
Independent 1-year mortality correlates were: not having a TAVI performed (HR = 76.92 95% CI: 3.47–1707; p = 0.006), moderate renal insufficiency (HR = 3.67, 95% CI: 1.29–10.44; p = 0.015) and a history of pulmonary edema (HR = 2.15, 95% CI: 1.15–3.99; p = 0.016). We found an interaction for the effect of TAVI on 1-year mortality according to FTSST (p = 0.049) (Table III).
Table III
One-year all-cause mortality, multivariate analysis
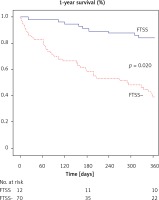
In the no-TAVI group, the multivariate Cox regression model performed revealed a success in FTSST to be the best predictor for 1-year mortality (HR = 0.18, 95% CI: 0.04–0.76; p = 0.019). There was also a trend towards history of pulmonary edema (HR = 1.81, 95% CI: 0.97–3.37; p = 0.06) and respiratory insufficiency (HR = 0.36, 95% CI: 0.12–1.03; p = 0.06) (Table IV, Figure 3).
Table IV
One-year mortality in no-TAVI group, multivariate analysis
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| History of pulmonary edema | 1.81 | 0.97–3.37 | 0.06 |
| Moderate renal insufficiency | 2.24 | 0.77–6.54 | 0.14 |
| FTSST success | 0.18 | 0.04–0.76 | 0.019 |
| Respiratory insufficiency | 0.36 | 0.12–1.03 | 0.06 |
Figure 3
Time-to-event curves for the primary endpoint according to the FTSST results among the no-TAVI patients
FTSST – Five-Time-Sit-to-Stand Test, FTSST – Success without assistance or with upper extremity support.
In the TAVI group, multivariate Cox analysis did not reveal any CGA parameter to be related to 1-year prognosis. Respiratory insufficiency (HR = 40.9, 95% CI: 6.52–257; p < 0.001) and moderate renal insufficiency (HR = 11.95, 95% CI: 1.57–90.90; p = 0.017) were the predictors of 1-year mortality.
Discussion
The main results of our study are: (1) TAVI was effectively associated with reduced 1-year all-cause mortality in presumably frail older patients suffering from severe aortic stenosis, and (2) FTSST was the best mortality predictor among the patients who did not undergo TAVI.
In the no-TAVI group, 1-year mortality was very high, which is consistent with previous literature [19, 20]. Only a few recent studies have reported long-term survival of untreated severe aortic stenosis, as the last dated palliative therapy was balloon aortic valvuloplasty (BAV) in the 1990s, when 1-year mortality was around 43% without [21] and 36% with [22] BAV. On the other hand, 1-year mortality in the TAVI group was 16%. Considering that our patients underwent CGA because they were presumed frail and unsuitable for TAVI, it is noticeable that this 16% 1-year mortality remains comparable with the 21.4–24% reported by the largest national TAVI registries [11, 23]. Beyond the TAVI procedure by itself, 1-year mortality predictors were history of pulmonary edema or moderate renal failure, as previously reported by others [24, 25], but none of the CGA parameters (Table III).
In the no-TAVI group, the FTSST was the best predictor for 1-year mortality. Poor mobility was already identified as a good predictor of poor outcomes and mortality in heart disease. For instance, the Gait Speed test – another measure of functional capacity – [26] is associated with both morbidity and mortality in older patients undergoing cardiac surgery [27]. In the TAVI population, the slowest walker and those unable to walk also presented higher mortality [28]. While loss of mobility may be inherent to the cardiac condition itself, our analysis showed the mobility assessment alone and not the usual cardiac markers to relate to mortality in the no-TAVI group. This result suggests that frail patients suffer more from a poor general mobility condition rather than a cardiac condition. We should acknowledge that every single patient from our cohort presented surgical aortic stenosis with severe symptoms (Table I), potentially explaining the fact that usual cardiac markers were not discriminant – and FTSST might relate to a worse prognosis in the general subset of older patients. The loss of mobility is also often related to weight loss and/or cachexia in older adults, yet mean BMI and MNA values were normal (25.7 ±5.1 kg/m2 and 7.9 ±2.3, respectively), and did not relate to prognosis.
Our patients are representative of patients from the beginnings of the TAVI procedure, and therefore very high-risk patients. In the new ESC recommendations [29], TAVI takes a greater part of the invasive treatment for symptomatic aortic stenosis. Indeed, TAVI will now be recommended from the age of 75, for patients with lowest surgical risk represented by a STS score ≥ 4% and a logistic EuroSCORE ≥ 10% (compared to 10% and 20% in 2012, respectively) [29]. Consequently, as TAVI will be considered in a larger number of lower risk patients, common medical comorbidities will become less prevalent and might lack accuracy in assessing long-term benefits, providing room for objective geriatric criteria.
The clinical interest of our results stems from showing evidence that TAVI improved overall survival in presumably frail patients, provided that the individual decision was approved by the CGA and heart team. Moreover, even though the present method was not meant to better identify patients who will benefit from TAVI, we highlight the possibility that investigating patients’ mobility might be a cornerstone in assessing further prognosis. Whether or not physical training should be encouraged in patients with loss of mobility remains to be investigated among patients with severe aortic stenosis.
Several limitations should be considered, the first being inherent bias due to it being a retrospective and observational non-randomized study, with a limited sample size. Secondly, our study assessed a very high-risk population, with severe symptomatic aortic stenosis, which was representative of the beginning of the TAVI procedure. Third, while there is evidence that frail older patients with TAVI exhibit higher mortality [30, 31], the proportion of TAVI patients was too low to allow us to study the impact of CGA parameters on prognosis. Finally, other prognostic markers might be investigated. The Charlson comorbidity index is a validated measure of 1-year mortality risk and disease burden [32]. A low serum vitamin D level in older adults has been demonstrated to be associated with cardiovascular risk [32], dementia [33] and all-cause mortality [34].
In conclusion, aortic valve replacement by a TAVI was associated with reduced 1-year mortality among presumed frail older patients with severe aortic stenosis. FTSST was an independent predictor of 1-year mortality in patients with severe aortic stenosis who did not undergo valve replacement.