Introduction
Malignant melanoma is one of the most malignant and deadly carcinomas in the human body, with an extremely high metastatic potential [1]. Its development is based on the malignant transformation of melanocytes, which implies its widespread distribution in the body [2]. Skin is the most commonly affected organ, but the tumour can also affect the uvea of the eyes [3], male and female genitalia (especially the vulva) [4], the gastrointestinal tract, the urinary system, etc. The primary risk factor for the development of the disease is chronic exposure to UV radiation as a component of solar radiation [1]. Australia and New Zealand are world leaders in terms of morbidity and mortality with rates of 54/100,000 and 5.6/100,000, respectively, for 2015 [5]. In Bulgaria, the morbidity rate for the same year is 6.5/100,000, and the mortality rate is 2.1/100,000. Treatment of malignant melanoma is complex, with the surgical method taking the lead.
Furthermore, a wide range of non-surgical treatment modalities is used, such as chemotherapy, targeted therapy, immunotherapy, and radiation therapy [6]. Radical re-excision of the tumour bed in the case of cutaneous malignant melanoma is a central part of the surgical treatment. It is performed within 4–6 weeks after the final diagnosis [7]. A part of the skin, including the scar from previous diagnostic excision of the primary tumour, together with the subcutaneous tissue, is surgically removed reaching the underlying fascia. This should be performed after the sentinel node biopsy (if there is any) to avoid disruption of lymphatic outflow from the area and worsened surgical outcome. For decades, surgeons performing the procedure have adhered to the 3–5 cm rule of wide radical excision in all directions to the scar from the initial biopsy. This leads to massive skin defects in the recovery, which permanently disrupts the function of the affected area. In this regard, many studies aiming to propose the narrowest possible re-excision margins have been conducted, in order to achieve local oncological radicalness. The Breslow melanoma thickness is one of the main prognostic indicators taken into consideration in all of the studies as mentioned above (Table I) [7–15].
Table I
Re-excision borders according to the thickness of malignant melanoma
| Thickness of the lesion according to Breslow‘s classification [mm] | Surgical borders [cm] |
|---|---|
| In situ | 0.5–1.0 |
| ≤ 1 | 1.0 |
| 1–2 | 1.0–2.0 |
| ≥ 2 | ≥ 2.0 |
Lentigo maligna (melanoma in situ) is an exception to the rules derived from these studies. Due to the low risk of recurrence after tumour removal, a re-excision with 0.5 cm resection margins can be performed, or the tumour can be removed by Mohs micrographic surgery: excising the tissue layer by layer and histologically examining each layer until completely removing the lesion [16, 17]. This microsurgery may be useful in removing lesions on the head, neck, arms, and feet for a better aesthetic and functional outcome, but there is still no convincing evidence of radicalness [18].
There is no definitive position on the exact size and extent of re-excision correlated to all thicknesses of cutaneous malignant melanoma in the studies mentioned so far that would guarantee sufficient oncological radicalness. We take the opportunity to present a clear and accurate answer to this question through our research.
We aim to test the hypothesis that the re-excision with a 2 cm margin in all directions to the scar from the previous biopsy of the primary tumour provides sufficient local control in patients with malignant melanoma of the skin.
Material and methods
This prospective descriptive study using STROBE methods in our patients with malignant melanoma of the skin was performed in conformity with the written approval of the Ethics Committee of the Medical University of Pleven, Bulgaria. All study participants signed an informed consent form.
A total of 151 patients were diagnosed and treated surgically at the Department of Plastic, Reconstructive and Aesthetic Surgery, Dr Georgi Stranski University Hospital, Medical University of Pleven, Bulgaria, in the period 2012–2016. Twenty-one cases were omitted from the study due to lack of sufficient data in the Bulgarian National Cancer Registry for the period from the beginning of 2012 to the end of 2019. Therefore, a total of 130 patients underwent statistical processing.
Surgical methods
Re-excision of the tumour bed after biopsy of cutaneous malignant melanoma was performed according to the criteria for good medical practice. The patients basically underwent re-excision within 2 cm margins in all directions to the scar from the previous biopsy of the primary tumour. For tumours of Breslow thickness < 1 mm, in cases of facial localisation, these margins were slightly narrowed. The surgical intervention started with skin incision, with the subcutaneous tissue cut obliquely to the sides until the underlying fascia was reached but remained intact. Thorough haemostasis and antiseptic lavage followed. Skin defects resulting from re-excision were often difficult to close normally, in which case we applied different types of non-free and free skin plastics. Finally, we used a sterile ointment bandage. The removed skin flap was sent for a permanent histological preparation to be evaluated for a residual tumour and invasion-free edges.
Statistical analysis
We used descriptive statistics employing STROBE methods for assessment of clinico-pathological features and stage distribution. Categorical features were summarised with frequencies and percentages. The statistical analysis of prognostic factors for local recurrence/death was performed with SPSS v.24.0.
Results
Distribution of the patients with malignant melanoma of the skin according to demographic, pathological, and clinical characteristics
The patient distribution according to main demographic, pathological, and clinical characteristics is shown in Table II.
Table II
Patient characteristics
| Prognostic factor | Number patients n = 130 | Percentage |
|---|---|---|
| Age (mean) [years]: | 61.6 | |
| Up to 40 | 15 | 11.5 |
| 40–65 | 51 | 39.2 |
| Over 65 | 64 | 49.3 |
| Male | 67 | 51.5 |
| Female | 63 | 48.5 |
| Histological type of melanoma: | ||
| Achromatic melanoma | 14 | 10.8 |
| Pigment melanoma | 116 | 89.2 |
| Pathological stage TNM 8*: | ||
| T0 | 8 | 6.2 |
| T1 | 22 | 16.9 |
| T2 | 30 | 23.1 |
| T3 | 32 | 24.6 |
| T4 | 38 | 29.2 |
| Stage 0 | 8 | 6.2 |
| Stage I A | 17 | 13.1 |
| Stage I B | 24 | 18.4 |
| Stage IIA | 12 | 9.2 |
| Stage IIB | 11 | 8.5 |
| Stage IIC | 18 | 13.7 |
| Stage III | 9 | 7 |
| Stage IV | 31 | 23.9 |
Distribution of the patients with skin malignant melanoma according to Breslow tumour thickness
Patients with malignant melanoma thickness over 4.1 mm, according to Breslow classification, had the highest frequency (28.0%), followed by the patients with thickness between 2.1 and 4.0 mm (25.0%) (Figure 1).
Figure 1
Distribution of the patients based on the thickness of skin malignant melanoma according to Breslow’s classification
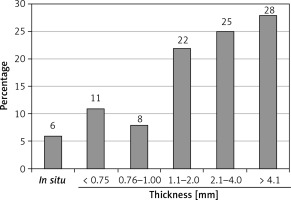
The mean thickness of malignant melanoma according to Breslow classification for the whole group of patients was 3.34 mm, with a range of values between 0 and 11.4 mm.
Distribution of the patients with malignant melanoma of the skin according to the presence of histologically proven residual tumour in the re-excised flap
Of all 130 re-excisions performed by us, only one (0.77%) had a histologically proven residual tumour.
Distribution of the patients with skin malignant melanoma according to the presence of local recurrence
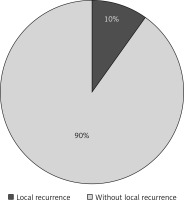
Local recurrences of malignant melanoma were observed in 13 patients, representing 10% of all 130 patients (Figure 2).
Survival in the patients with malignant melanoma of the skin
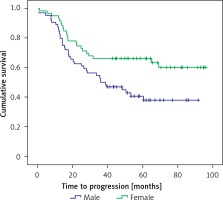
The 5-year survival in our patients was 63%. Looking at the overall survival of patients by sex, we found that it was significantly higher in the women than in the men (log-rank = 6.150, df = 1, p = 0.013) (Figure 3).
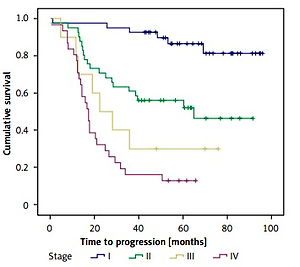
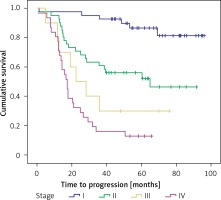
There was a significant difference in the overall survival depending on the clinical stage of cutaneous malignant melanoma; it was highest in the first stage (log-rank = 54.195, df = 3, p < 0.001) (Figure 4).
Discussion
The results of this prospective study show that the male patients tended to predominate (51.5%), which is in line with global trends [19, 20]. The differences encountered by us in comparison to data in the research literature [21–25] are related to the higher mean age of 61.6 years (range: 17–91 years) and the relatively high percentage of patients with Breslow thickness over 4.1 mm (28.0%) and in stages III and IV (30.9%). Considering the last two negative facts, we committed to resolving the issue with the resection margin perimeter to the scar of the previous melanoma biopsy, which would allow sufficient local oncological radicalness. We performed a detailed literature analysis along with an assessment of the latest updates and guidelines for the management of cutaneous malignant melanoma [11–14, 20, 26–34]. We selected the option for a more limited -excision with 2 cm margin in all directions to the biopsy scar. A similar gentle local surgical intervention applied in the treatment of congenital subungual nevus with high malignancy risk was reported by López et al. [35]. We found that despite the greater Breslow thickness and the more advanced stage of skin melanoma, this volume of re-excision was completely sufficient and safe. This fact was confirmed by the histological examination of all re-excision specimens. The histological evaluation identified only 1 (0.77%) patient with a residual tumour. Exceptions to this volume of re-excision should be rarely allowed: only for lesions with Breslow thickness < 1.5 mm and lesions with facial localisation, in which case the borders should be about 1–1.5 cm. The minimum observation period for our patients after re-excision was 3 years, and the maximum was 7 years. In the last 2 years of monitoring no new local recurrences have been observed. This indicates that time as a factor is not an essential determinant of their occurrence. We discovered local recurrences in 10% of cases for the entire observation period, which is two times higher than cited in the research literature (3–5%) [36–38]. Examining in detail the group with local recurrence, we found that relapse was seven times more frequent in patients with achromatic melanoma than in patients with pigmentary melanoma. The other two factors directly influencing the onset of local recurrence are late initial diagnosis and advanced clinical stage. We determined that reduced volume of re-excision was not a cause of a local recurrence, because in 99.23% of cases there was no residual tumour in the histological examination of the flap. Most likely, it is related to a secondary invasion of the re-excision scar by free-moving melanoma cells with the blood flow [39].
Survival in the men and in the patients with advanced skin malignant melanoma in our study was significantly lower, which is in line with the data from two large-scale studies conducted in Brazil [40] and Europe [41]. However, the 5-year survival rates of 95.7% and 85%, respectively, are substantially higher than the 63% survival rate established by us. The negative trend in our patients’ survival rate is mostly related to the late-stage initial diagnosis of the disease.
In conclusion, the diagnosis of cutaneous malignant melanoma in our patients was made at an older age, and the frequency of advanced-stage cases was higher than described in the literature.
Re-excision with a 2 cm margin in all directions to the scar from the previous biopsy of the cutaneous malignant melanoma is sufficient to achieve local surgical radicalness in the treatment of the disease without compromising oncological survival.
Higher local recurrence rates established in our study in comparison to the literature data are not due to a lower volume of re-excision but presumably result from a secondary invasion of the re-excision scar by free-moving melanoma cells with the blood flow.
Male sex and advanced stage are indicators worsening the prognosis of malignant melanoma, the second of which was particularly indicative for the patients included in this study.