Introduction
In the past few years, treatment of multiple myeloma (MM) has undergone a deep change for the employment of novel treatment comprising proteasome inhibitors (PIs), immunomodulatory drugs, epigenetic therapy, and monoclonal antibodies [1–3]. This event, combined with the frequent employment of autologous stem cell transplant and the progress in the knowledge of MM biology [4], has led to improved overall survival for MM subjects [5].
Bortezomib is the first of a novel group of drugs known as PIs [6] and is a first-line chemotherapeutic in therapy of newly diagnosed MM (NDMM).
Bortezomib is a boronic acid dipeptide which reversibly blocks the 20S catalytic subunit of the 26S proteasome. Proteasome inhibition has been demonstrated to block crucial cell pathways, comprising the ubiquitin-proteasome pathway and nuclear factor κB (NF-κB), JNK and p53 function, to cause cell death. Multiple myeloma cells are more susceptible to the action of the drug, undergoing cell death more promptly than normal cells. Moreover, bortezomib can induce G2M phase cell cycle arrest and tubulin polymerization, and axonal transport inhibition [7, 8].
The use of bortezomib treatment was associated with the onset of peripheral neuropathy as a frequent, dose-limiting collateral effect [9]. Several studies describe elevated occurrence of peripheral neuropathy (more than 70%) after bortezomib treatment [10]. Moreover, numerous studies have demonstrated that although up to 75% of subjects with bortezomib-induced painful neuropathy (BIPN) had resolution of symptoms within 2–3 months of treatment termination or dose modification [11], 25% of BIPN subjects continue to have symptoms also after dose reduction or drug interruption (13–20 months) [12].
BIPN is generally a distal symmetric neuropathy which affects both large and small fibers although in many cases symptoms of small-fiber damage predominate. BIPN is described as a condition characterized by several symptoms such as hypoesthesia and heat hypersensitivity with poor motor involvement [13]. Its main characteristic is presence of grave neuropathic pain which is often devastating. BIPN is not treatable with any treatment and critical forms require bortezomib suspension [14, 15].
Nerve conduction study (NCS) can be used to make a diagnosis of BIPN [16, 17], but generally this technique can demonstrate alterations of the large nerve fibers and not those implicating the small fibers.
Modifications in the peripheral autonomic nervous system are an initial expression of distal small fiber neuropathy [18]. Sudomotor alteration is one of the earliest anomalies in small fiber disorders. Sweat glands are innervated by unmyelinated cholinergic sympathetic C-fibers and several reports have demonstrated a decrease in the epidermal C-nerve fibers in diabetic subjects with small fiber neuropathy [19, 20]. Hence, evaluation of sudomotor function may be an efficacious method to study peripheral small fiber neuropathy in different settings of patients [21].
Sudoscan is a new and modern evolution of electrophysiological methods aiming to provide a non- invasive and quantitative evaluation of the sudomotor function [22, 23]. It measures in microsiemens (µS) the electrochemical skin conductance derived from the reaction between the sweat chloride and the nickel electrodes. Recently, Sudoscan has been considered a helpful tool in the assessment of small fiber neuropathy (SFN) caused by diabetes [22], mitochondria dysfunction [24], and amyloid deposition [25].
The main objective of this study was to evaluate sensitivity of the NCS and Sudoscan, for detecting early peripheral nerve involvement in MM patients after treatment with bortezomib. Moreover, this study aims to evaluate whether this measure could be used as a reliable tool to detect longitudinal changes in the small fiber function in these patients after bortezomib treatment. The results of our study would seem to confirm the possibility that Sudoscan may be a useful means of evaluating the BIPN.
Material and methods
This study was carried out in accordance with the principles of the Declaration of Helsinki and the Local Ethic Committee approved this study protocol before the initiation of any study-related procedures; informed consent was obtained from every participant.
This study was conducted from May 1, 2017 to January 20, 2019. A total of 18 NDMM patients were studied, 10 (55.5%) men and 8 (44.5%) women. The median age was 70.0 years (range: 39–87). International Staging System (ISS) I, II, and III disease stages at diagnosis were prevalent in 5 (27.8%), 6 (33.3%), and 7 (38.9%) of the participants, respectively.
Exclusion criteria were amputation of legs or arms, symptoms limited to the upper extremity due to cervical radiculopathy or upper extremity entrapment neuropathy, implanted electrical devices, diabetes mellitus, and previous peripheric polyneuropathy (PPNP) history.
The paraprotein class was immunoglobulin G (IgG) in 13 (72.2%) patients, and IgA in 5 (27.8%) patients. Light chain was κ in 10 (55.6%) patients, and λ in 8 (44.4%) patients.
At baseline routine laboratory tests consisted of complete blood count, and blood chemistry including β2 microglobulin (Beta2m), serum albumin, calcemia, and renal function. Physical examination and skeletal X-rays were also performed. Fourteen (77.8%) subjects had lytic bone lesions, while only 4 (22.2%) did not present them.
Five (27.7%) patients received bortezomib, thalidomide and dexamethasone (VTD) chemotherapy, while 13 (72.2%) were treated with the bortezomib, melphalan and prednisone (VMP) regimen.
At baseline and after 6 months of treatment patients were submitted to a clinical measure of pain and neuropathy. The three scales employed were the Douleur Neuropathique en 4 Questions (DN4), the Total Neuropathy Score (Clinical) (TNSc), and the Pain Intensity Numerical Rating Scale/PINRS.
The full version of the DN4 comprises 7 self-report items and 3 clinical examination questions. Scores range from 0 to 10 and a score of 4 or higher is considered positive for neuropathic pain [26].
The TNSc combines data acquired from quantitative sensory examinations, and scoring of signs, thus supplying a single measure to evaluate neuropathy [27].
For the PINRS the patients are requested to classify their pain with a numerical value indicating its intensity. The PINRS scale ranges from 0 to 10, with 10 indicating the worst possible pain [28].
The electrochemical skin conductance (ESC) was evaluated in the MM subjects at baseline and after 6 months of treatment, with the Sudoscan device (Impeto Medical: Paris, France) [29].
All sudomotor function tests were performed in a quiet and temperature-controlled autonomic laboratory with ambient air humidity (room temperature: 22–23°C, room humidity 40–60%). Subjects were in an upright position during the Sudoscan testing. The electrochemical principle involves the induction of a chloride-based electrochemical current after activation of sweat glands by a low-voltage current (< 4 V) [30]. Due to the isolator function of the corneal stratum of the epidermis, it is expected that the measured net current corresponds to the local sweat response. The ESC in microsiemens (µS) that represents the chloride ion current is calculated by the ratio of extrapolated current and constant direct current. ESC was measured at both hands and feet by placing the palms and soles on stainless steel electrodes for 2 min. The measurement was repeated twice, and the average ESC was calculated. Low ESC indicates a high risk of a somatosensory neuropathy [31]. Based on a previous study of Vinik and colleagues, an ESC of > 70 µS (feet)/> 60 µS (hands) is considered to indicate normal sudomotor function while an ESC of 50–70 µS (feet)/40–60 µS (hands) and of < 50 µS (feet)/< 40 µS (hands) is suggestive of moderate and severe sudomotor dysfunction respectively [29].
NCS consisted of sensory nerve conduction of the sural and ulnar nerve and motor nerve conduction of the peroneal, tibial and ulnar nerve. The voltage applied to subjects for the nerve conduction study was about 90–150 V.
NCS was conducted according to internationally accepted standards, and the 2SD lower limit of normal of local reference values was used for statistical testing and to determine the percentage of abnormal measurements [32].
Power and sample size
Sample size calculation is based on the primary outcome, which is the incidence of multiple myeloma. We assume for this study a known population incidence of 0.00875% (since in Italy the incidence is estimated to be 8.75 new cases per 100,000 inhabitants) and expected incidence of 9% (this is the anticipated incidence of MM for our study group). In order to guarantee a power of power of 80%, and a two-sided significance level of 5%, the sample size calculation suggests that the minimum number of subjects is 8. In our study we enrolled 18 patients.
Statistical analysis
Numerical data are expressed as mean and standard deviation (S.D.) and categorical variables as number and percentage. The non-parametric approach was used since the numerical variables were not normally distributed, as verified by the Kolmogorov-Smirnov test. The Wilcoxon test was applied to perform comparisons between times of observation (baseline vs. 6 months) with reference to numerical parameters (DN4, PI-NRS and TNSc); the McNemar test was applied for dichotomous data (NCS and Sudoscan). The Spearman correlation test was applied to assess the existence of any significant interdependence between analyzed parameters. Finally, the Pearson χ2 test was used to analyze differences between the two techniques, NCS and Sudoscan, with reference to the percentages of alteration, both at baseline and after 6 months of treatment.
Statistical analyses were performed using IBM SPSS for Windows, Version 22 (Armonk, NY, IBM Corp.). A p-value smaller than 0.05 was considered statistically significant.
Results
After treatment the overall response rate to treatment was 83.3%. Complete responses were observed in 5 of 18 patients (27.7%), very good partial responses in 10 (55.5%) subjects, and 3 (16%) patients experienced progression of disease.
At baseline the NCS profiles showed that only the mean sural nerve sensory nerve action potential (SNAP) amplitude was below the 2SD lower limit of normal (LLN; in 3/18 or 16.7% of patients), based on normative values generated in our Laboratory of Clinical Neurophysiology, while at the same time we found an alteration of Sudoscan profiles in 2 patients (11.1%) (Figure 1).
Figure 1
NCS and Sudoscan profiles at baseline and after treatment. “Normality” and “Alterations” indicate, respectively, the absence and the presence of alterations found in NCS and Sudoscan
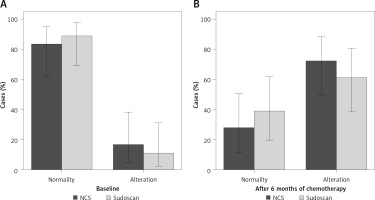
After 6 months of treatment, the NCS profiles (especially mean sural SNAP) were altered in 13 (72.2%) patients, and the Sudoscan profiles were modified in 11 (61.1%) subjects (Figure 1).
Employing the McNemar test, the NCS results at baseline and after 6 months were significantly different (p = 0.002). Analogously, Sudoscan analysis before and after chemotherapy was statistically different (p = 0.004).
Using the Wilcoxon test for comparison between time points (baseline and after 6 months) of the scales for the study of neuropathy, we observed a significant change after therapy compared to baseline for the DN4 (Z = –3.071, p = 0.002), for the PINRS (Z = –2.612, p = 0.009), and for the TNSc (Z = –2.992, p = 0.003). These results indicate that bortezomib therapy caused an initial polyneuropathy both clinically and neurophysiologically.
We found a significant correlation between the results obtained with the NCS study or Sudoscan and the scales for the evaluation of neuropathy (Figures 2, 3 – box plot). In particular, using the Spearman test, we found that at baseline NCS correlates with the results of DN4 (rs = 0.578, p = 0.01), PINRS (rs = 0.482, p = 0.04), and TNSc (rs = 0.593, p = 0.009). The correlation was maintained even after 6 months of treatment but only between NCS and DN4 (rs = 0.485, p = 0.041).
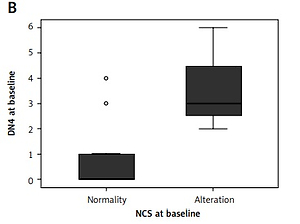
Figure 2
Box plot of DN4 values and PINRS values compared with changing in NCS before and after treatment with bortezomib. “Normality” and “Alterations” indicate, respectively, the absence and the presence of alterations
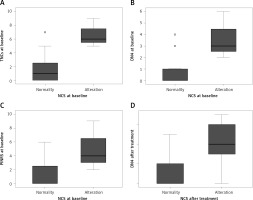
Figure 3
Box plot of DN4, PINRS and TNSc values compared with changing in Sudoscan before and after treatment with bortezomib. “Normality” and “Alterations” indicate, respectively, the absence and the presence of alterations
A significant and positive correlation was also found between the baseline values obtained with Sudoscan and the scales for neuropathy, specifically with the DN4 (rs = 0.469, p = 0.049), with the PINRS (rs = 0.500, p = 0.03) and with the TNSc (rs = 0.528, p = 0.024). A better correlation was found between Sudoscan and neuropathy scales after treatment with respect to NCS. Indeed, after therapy there was a significant correlation between Sudoscan and DN4 (rs = 0.768, p = 0.001), with PINRS (rs = 0.824, p = 0.0001), and with TNSc (rs = 0.751, p = 0.0001). This different correlation could indicate that Sudoscan is an adequate method to highlight an initial polyneuropathy. No correlation emerged between NCS or Sudoscan and the biological variables considered (Hb, creatinine, LDH, Beta2m, Ig class, light chain, presence of lithic lesions, stage of disease). The only exception was a negative correlation between NCS after 6 months of therapy and calcemia values (rs = –0.574, p = 0.013).
Finally, using the Pearson χ2 test, no statistically significant differences were found between the two techniques used, NCS and Sudoscan at baseline (χ2 = 0.232, p = 0.630) and after 6 months of treatment (rs = 0.500, p = 0.480).
No difference was detectable for the variables examined between patients subjected to VTD and those subjected to the VMP regimen.
Discussion
To the best of our knowledge, this study is the first to assess sudomotor dysfunction in patients with MM using a Sudoscan. The primary research objective was to evaluate whether Sudoscan could identify variations of its parameters in MM patients at diagnosis and after bortezomib treatment. The secondary objective was to evaluate the diagnostic validity of the results and establish the clinical usefulness with respect to conventional NCS.
In our study, we demonstrated for the first time the non-inferiority of the Sudoscan compared to the NCS, in the diagnosis of bortezomib-induced neuropathy in MM patients, evidencing that at the baseline and after therapy there are no statistically significant difference between the two techniques.
Indeed, there seems to be better agreement between Sudoscan and scales for the evaluation of neuropathy with respect to the correlation between scale used and NCS. In fact, after treatment the NCS correlates only with the TNSC, while the Sudoscan maintains a significant correlation with all the scales used. These data could confirm the validity of the technique for the evaluation of bortezomib neuropathy in patients with MM.
The initial identification of neuropathy in clinical practice has been a challenging problem [33]. Conventional assessment techniques, such as the tuning fork and the 10-g monofilament, are subjective and necessitate the patient’s collaboration [34, 35]. Generally, a conclusive neuropathy diagnosis is made based on examinations such as NCS, which reveal the alteration of large myelinated fibers. However, the findings could be sometimes normal in subjects with early or small fiber prevalent disease [36].
Alterations in peripheral autonomic nervous system conduction are a precocious expression of distal small fiber neuropathy, as sudomotor dysfunction is the initial demonstrable neurophysiologic modifications in distal small fiber neuropathies. For this reason, the American Association of Clinical Endocrinologists proposed sudomotor evaluation as the best tool to evidence early peripheral autonomic neuropathy in diabetic patients.
Currently, there are several techniques of evaluating sudomotor function [37], but unfortunately, none are appropriate for application in daily clinical practice due to the needs of highly prepared operators, protracted investigating time, expensive apparatus, and difficult subject training [38]. The possible employment of Sudoscan seems to resolve all these issues, as it can be executed in a few minutes, does not need any subject collaboration or specific preparation of the operators, and it is absolutely noninvasive.
BIPN is characteristically sensory, and the main symptoms are itchiness, hyperesthesia or hypoesthesia, pain or burning sensations [11, 39]. However, motor polyneuropathy from bortezomib is infrequent and generally appears after sensory polyneuropathy.
The pathogenetic mechanism of BIPN has not yet been distinctly described. Several results have indicated the existence of genetic factors [40]. Certain pathways might have a central action in BIPN, such as NFκB, DNA repair, and mitogen-activated protein kinase-mediated signaling [41–43]. Bortezomib has also been demonstrated to act on mitochondria, leading to a decrease in mitochondrial membrane potential and augmented reactive oxygen production (ROS) in vitro [44]. These observations and the well-known alteration of oxidative stress in MM patients [45] support the possibility that in vivo ROS scavenging may be a successful target in blocking BIPN.
Finally, there are signs of calcium dysregulation in bortezomib-caused cell death, with bortezomib provoking temporary intracellular ER calcium release, causing a mitochondrial-calcium influx and caspase activation [46]. This temporary modification may modify the membrane potential of neurons and provoke spontaneous action potentials, generating pain signaling after bortezomib treatment [47].
In this regard, our detection of a negative relationship between serum calcium levels and NCS after treatment with bortezomib (–0.574, p = 0.05) seems to be worthy of in-depth analysis.
Recent reports have demonstrated the diagnostic validity of ESC evaluation, with a sensitivity of 73% to 78%, and specificity of 62% to 100% to evidence PPNP in diabetic patients as well as idiopathic polyneuropathy (PN) and oxaliplatin or paclitaxel chemotherapy-induced PN [48–50], and previous studies assessing the Sudoscan risk score registered 65–92% sensitivity and 49–80% specificity to evidence cardiac autonomic neuropathy [51].
Our results suggest that sudomotor function, assessed by Sudoscan, can be considered for the diagnosis of bortezomib-induced neuropathy. Sudoscan analysis is painless, can be conducted in a few minutes, and neither special patient preparation nor specially trained operators are needed. It is objective, reproducible, and surely easier than the traditional NCS.
However, our study has some limitations. In fact, the number of subjects studied is too small to be able to draw definitive and generalizable conclusions, and a longer follow-up of the patients would be appropriate. Nevertheless, in view of the pathophysiology of BIPN, which suggests the predominant participation of small fibers in the onset of the disease, it is probable that subsequent studies conducted on a larger population will demonstrate even better results regarding the sensitivity and specificity of Sudoscan compared to the NCS.
So, Sudoscan may become a novel technique for diagnosis of neuropathy in daily activity, and a useful help to manage the therapeutic interventions in MM.