Introduction
Chemokines, in other words chemotactic cytokines, primarily regulate the motility and migratory properties of cells [1]. In the tumor microenvironment, they play a crucial role in the tumor cells’ survival, growth and proliferation. It is also known that they strongly participate in tumor progression according to their proangiogenic and prometastatic action. Moreover, chemokines have a confirmed modulatory effect on the immune cells’ activity, both present in the tumor microenvironment and circulating in peripheral blood. Among others they play a crucial role in leukocyte infiltration into the tumor; therefore they control what type of effector cells (CD4+, CD8+, macrophages, NK cells) are required [2–4]. Among many chemokines that are involved in cancer progression, not widely investigated in patients with ovarian cancer, those mentioned below are worth distinguishing.
CX3CL1, or fractalkine, is the only member of the CX3C subfamily of chemokines and exists in two forms. The membrane-anchored form mediates strong cell adhesion while the soluble form acts mainly as a chemoattractant for T cells, NK cells, dendritic cells and CD14 positive monocytes [5–7]. CX3CL1 is a specific ligand of the CX3CR1 receptor [8]. The expression of CX3CL1 and CX3CR1 has been established in different cancers such as breast, prostate, pancreatic and ovarian cancer [9–13].
CXCL12, also known as stromal-derived factor 1α (SDF-1α), is a chemokine member of the angiogenic CXC family strongly associated with tumor growth, metastasis, angiogenesis, and chemoresistance. Moreover, it also plays an important role in the communication between tumor cells and their stromal cells in the tumor microenvironment. CXCL12 and its receptor CXCR4 have been found to be overexpressed in a variety of solid tumors: breast, colon, prostate, melanoma, and ovarian cancer [4, 14–16].
CXCL1, also called growth regulated oncogene-α (GRO-α), another member of the CXC family, interacts with CXCR2. This protein is best known as an effective chemoattractant guiding neutrophils through venular walls. However, its involvement in tumor cell proliferation and migration as well as cancer progression, although less known, was also described. GRO-α was found to be expressed at a high level in a series of human cancers such as breast, gastric, bladder and ovarian [6, 17, 18]. GRO-α together with CXCL8 causes robust endothelial cell proliferation, tube formation and migration in a mouse model of ovarian cancer [19].
CCL20 is a small protein, with CCR6 as its cellular receptor [20]. Previous studies demonstrated that TNF activates the nuclear factor-κB signaling to induce proinflammatory chemokines, CCL20 being one of them [21]. Serum concentration of CCL20 may be elevated in women with ovarian cancer [22].
IL-17F is a member of the IL-17 family, mainly produced by T-helper (Th17) cells and macrophages, sharing the strongest amino acid sequence with IL-17A. Il-17F participates in allergic inflammation in the lung, and intestinal inflammation in vivo [23].
Ovarian cancer is still the leading problem in gynecological oncology. It accounts for about 25% of gynecological malignant neoplasms, but is responsible for almost 50% of deaths caused by these malignancies. Almost 75% of patients are diagnosed in the advanced stage of the disease (stages III and IV) due to the absence or nonspecific clinical symptoms at the beginning and the lack of screening methods for diagnosis. The gold standard in the diagnosis of pelvic masses is still a bimanual gynecological examination supplemented by transvaginal sonography and serum markers. The most common serum marker used is CA125. However, it is not specific for this disease. It is elevated in about 80% of patients with ovarian cancer, but also in about 60–80% of cases of liver cirrhosis, up to 20% of patients with endometriosis, benign and borderline ovarian tumors and in 1–5% of healthy women [24]. In the last few decades, many serologic biomarkers have been evaluated during the diagnosis of ovarian cancer (e.g. human epididymis protein 4 (HE4), soluble mesothelin-related peptides (SMRP), activin, inhibin, osteopontin, leptin, IL-6, IL-8), including those based on tumor-host immunologic interactions (various cytokines and antibodies) [24]. Up till now only HE4 (ROMA) has started to be applied in clinical practice.
This study aimed to investigate the serum levels of selected cytokines/chemokines in patients with ovarian cancer or benign ovarian tumors and their potential use in preoperative diagnosis of adnexal masses.
Material and methods
Patients
Our study, approved by the local Ethical Committee, was carried out in the Department of Operative Gynecology and Gynecologic Oncology of the Polish Mother’s Memorial Hospital – Research Institute, Lodz, Poland. We checked the patients with a pelvic mass suspected to be an ovarian tumor admitted to the department. Patients with a history of any previous malignant neoplasia or gynecologic operation, transplantation, autoimmunologic disease, diabetes, thyroid problems or any signs of infection were excluded from the study. After operative treatment and histopathologic examination, we finally collected 59 patients for further analysis: 17 women with epithelial ovarian cancer (mean age: 60.4 years; range: 37–82 years) and 42 age-matched women with benign ovarian tumors of epithelial origin (mean age: 54.2 years; range: 30–80). The stage of ovarian cancer was established after laparotomy and pathologic examination, according to the International Federation of Gynecology and Obstetrics (FIGO) classification and protocol [25]. All tissues removed during surgery were examined by a pathologist: tumor grade, histological type and the presence of metastases were established, according to FIGO and WHO classifications [25, 26].
The clinical and histopathological data of ovarian cancer patients are listed in Table I. In our study group, 7 (41%) women were diagnosed and operated on in the early stage of the disease (stages I and II) and 10 (59%) patients were suffering from advanced ovarian cancer (stages III and IV). The most common was serous (53%), then mucinous (18%) and clear cell carcinoma (12%). The mean age of ovarian cancer patients and women with benign ovarian tumors did not differ significantly (60.4 ±12.4 vs. 54.2 ±11.9). The benign ovarian tumors were mainly serous (45%), endometrial (29%) and mucinous (17%) (Table II).
Cytokine/chemokine determinations
Peripheral venous blood from patients with ovarian tumors was obtained in the morning of the day of the surgery and transported to the laboratory within 1 h of collection. Body fluid samples were centrifugated at 1500 x g for 10 min and then stored at –80ºC until analyzed. We measured a panel of 5 cytokines/chemokines – CX3CL1 (fractalkine), CXCL1 (GRO-α), CXCL12 (SDF-1), CCL20 (MIP-3α), and IL-17F – using the chemiluminescence method with multiplexed bead based immunoassay (Panomics, Inc., USA), which allowed simultaneous analysis of numerous analytes in one sample. The results are presented in pg/ml. The CA125 level in the blood samples was measured using Elecsys CA125 II assay (Roche Diagnostics, Indianapolis, USA), in accordance with the manufacturer’s instructions and expressed in U/ml.
Statistical analysis
For the continuous data we calculated mean, median, standard deviation (SD) and standard error of the mean (SEM). Verification of the distributions was made with the Shapiro-Wilk test. To compare the differences among groups we used the Mann-Whitney U test. The differences were considered statistically significant for p-value < 0.05.
Results
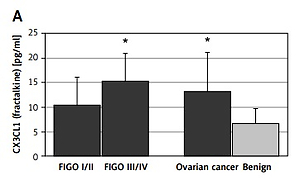
CX3CL1 (fractalkine) was significantly elevated in sera of ovarian cancer patients compared to women with benign ovarian tumors (13.3 ±7.9 pg/ml vs. 6.7 ±3.0 pg/ml, p < 0.005). When FIGO stage was taken into consideration we found that the mean level of serum CX3CL1 in advanced ovarian cancer (FIGO III/IV) was 15.3 ±8.7 pg/ml and was significantly higher than in the group of benign ovarian cysts (p < 0.01); in the early ovarian cancer group (FIGO I/II) it was 10.5 ±5.7 pg/ml and did not differ significantly from the benign group (Figure 1).
Figure 1
Serum concentrations of selected chemokines and CA125 in patients with ovarian cancer or benign ovarian tumors. Data are presented as a means pg/ml ± SD (CA125 as means U/ml ± SEM). Statistical significance: *early, advanced or overall ovarian cancer vs. benign ovarian tumors, p ≤ 0.05
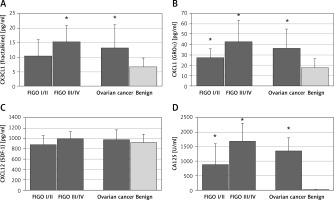
The levels of CXCL1 (GRO-α) were also significantly higher in sera of patients with ovarian cancer than in the benign group (36.5 ±18.4 pg/ml vs. 17.6 ±8.7 pg/ml, p < 0.01). Moreover, both early and advanced ovarian cancer patients (FIGO I/II: 27.5 ±8.6 pg/ml; FIGO III/IV: 42.7 ±20.6 pg/ml) had significantly higher levels of CXCL1 than women with benign ovarian tumors (p < 0.05) (Figure 1). The same differences were observed in the levels of the standard ovarian cancer serum marker CA125; in both groups of ovarian cancer patients its amount was significantly elevated (Figure 1).
Serum concentrations of CXCL12 (SDF-1) were similar in all investigated groups of patients (Figure 1). Only 2 (3.4%) patients had serum titers of CCL20 (MIP-3α) and 12 (20.3%) of IL-17F over the lower limit of detection (2.0 pg/ml and 1.06 pg/dl, respectively), so these cytokines were excluded from the analysis.
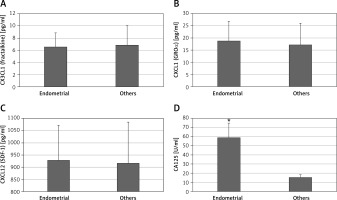
The main limitation of CA125 is that its serum concentration is elevated not only in cases of ovarian cancer but also in other conditions such as endometriosis. In our patients with endometriotic ovarian cysts serum CA125 levels were significantly higher than in women with other benign ovarian tumors (59 ±16 vs. 15 ±6 U/ml, p < 0.05) (Figure 2). All analyzed chemokines – CX3CL1 (fractalkine), CXCL1 (GRO-α), CXCL12 (SDF-1) – had similar serum titers in patients with endometriotic vs. other benign ovarian cysts (Figure 2).
Discussion
Inflammation is an essential component of the tumor microenvironment of ovarian cancer. The chemokines and cytokines released by cancer and stromal cells are the mediators of inflammation. Chemokines are primarily responsible for support of tumor cell survival and proliferation as well as for recruitment of immune cells from blood to the tumor tissue. They also have a strong pro-angiogenic effect required for tumor growth and progression. The presence of cytokines and chemokines in cancer patients is not connected with the tumor microenvironment only; they are also present in the circulation, since they are secreted into the surrounding tumor blood vessels [2, 3]. Some reports indicate that circulating chemokines could be considered as useful cancer prognostic markers [27, 28]. Therefore, in this study we tested the usefulness of selected chemokines in the preoperative diagnosis of ovarian tumor.
The available data on CX3CL1 involvement in ovarian tumors are extremely limited. Published papers have reported that specimens of both ovarian carcinoma and benign tumors expressed CX3CL1 and its receptor [7, 8, 13]. However, no data on the serum level of this chemokine in patients suffering from ovarian cancer or benign ovarian tumors have been reported. Our results clearly showed that CX3CL1 is elevated in sera of ovarian cancer patients in comparison to benign ovarian tumors, which points to its role in cancer development and/or progression. A higher level of CX3CL1 was also found in the ascites of patients with ovarian cancer than in peritoneal fluid of women with benign gynecologic diseases [29]. One of the proposed explanations of the involvement of CX3CL1 in ovarian cancer development is its high ability to induce cell proliferation via CX3CR1, which was well documented in the in vitro model of human epithelial ovarian carcinoma cell lines [7, 29]. Thus, a possible role of CX3CL1 in ovarian carcinogenesis was also proposed by Kim et al. [29], who found that normal ovarian surface epithelium was negative for CX3CR1, while this specific receptor was highly expressed in epithelial ovarian carcinoma, which enables the response of cancer cells to circulating CX3CL1. Furthermore, some authors also found that CX3CL1 presence in malignant ovarian tissue positively correlated with levels of Ki-67 and GILZ, two markers of proliferation in malignant ovarian epithelial cells [7]. Involvement of CX3CL1 in cancer progression is also connected with its ability to induce cell motility. The proinvasive and promigratory character of CX3CL1 has been demonstrated against the human breast cancer cell line MDA-MB-231 [30] and ovarian cancer cell lines SK-OV-3 and Caov-3 [29] among others. Involvement of CX3CL1 in facilitating epithelial ovarian cancer cells to migrate to the peritoneum and to adhere to the peritoneal wall was also documented [13].
We also observed that the serum level of CXCL1 was significantly higher in the ovarian cancer patients than in those with benign tumors. The elevated concentration of CXCL1 in sera of cancer patients in comparison to normal control subjects [31–33] or benign tumors [27] was found by others, as well. Thus, CXCL1 could be considered as a helpful serological marker, since it provides 97% specificity and 92% sensitivity for discriminating ovarian cancer patients from women with benign pelvic masses, whereas sensitivity and specificity of CA125 were significantly lower [27]. A high level of CXCL1 was also found in ascites and tumor tissue of ovarian cancer patients [32, 33]. Moreover, data presented by Couderc et al. [34] clearly showed that the concentration of CXCL1 in the sera of patients with ovarian adenocarcinoma resistant to platinum was higher than in patients with platinum-sensitive tumor. Therefore, CXCL1 was considered as a predictor of ovarian cancer response to chemotherapy. Similarly to our study, it was also reported that the serum level of CXCL1 was not increased in endometriosis patients [35]. It is known that CXCL1 exerts multiple effects directly on the cancer cells as well as on stromal cells such as fibroblasts and immune cells promoting tumor development, progression, and metastasis [2]. It strongly induces the proliferation of tumor cells, as was clearly shown in the studies with various ovarian cancer cell lines overexpressing or lacking CXCL1 or treated with exogenously added chemokine [36]. CXCL1-induced cell proliferation was dependent on its effect on the intracellular signaling pathway (mitogen activated protein kinase, MAPK) involved in cell division and nuclear factor κB responsible for cell survival [37, 38]. Meanwhile, silencing of CXCL1 in ovarian cancer cells resulted in inhibition of cell growth, invasiveness and aggressiveness [36, 38]. The pro-metastatic character of CXCL1 was well documented by studies by Yung et al. [38], who found that this chemokine and interleukin 8 are the predominant factors present in the cancerous omentum and they allow cancer cell seeding and growth in the peritoneal cavity. Moreover, CXCL1 was reported to stimulate fibroblast senescence to reprogram the stromal microenvironment, which in turn promoted the malignant transformation of epithelial cells [32]. The importance of CXCL1 in tumor progression may also be connected to its influence on immune cells. Among others, this chemokine controls migration and infiltration of neutrophils to the site of tumor [2, 17]. Tumor-associated neutrophils are directly implicated in the invasion and metastasis of cancer cells, mainly through remodeling of the basal membrane barrier and enhancement of angiogenesis [39, 40].
Thus, CX3CL1 and CXCL1 were both elevated in sera of ovarian cancer patients when compared to women with benign ovarian tumors. Moreover, these chemokines’ serum levels did not differ in women with endometriotic vs. other benign cysts. Thus, they might be useful in preoperative differential diagnosis of ovarian tumors, especially considering their behavior in patients with endometriosis. Actually, application of HE4 into the diagnostic protocol of ovarian tumors made it possible to verify the majority of false positive results of CA125 in patients suffering from endometriosis [41]. According to available data, levels of CA125 are elevated in approximately 67% of cases of endometriosis while HE4 levels are increased only in 3% of them [42]. Nevertheless, still some endometriomas are preoperatively suspected of potential malignancy. In patients with benign ovarian tumors CA125 and HE4 are elevated in 29% and 8% of cases, respectively. False positive results of CA125 and HE4 occur respectively in 20% and 8% of serous tumors, 18% and 13% of mucinous benign tumors, 26% and 8% of fibromas, and 37% and 10% of inflammatory lesions [42]. Consequently, despite improvement of the diagnostic protocol after implementation of HE4, before surgical treatment some benign ovarian tumors are constantly considered as malignant. Therefore, discovery and application of new biomarkers might be needed to increase the specificity of preoperative diagnosis of ovarian tumors.
Furthermore, the present diagnostic protocol of ovarian cancer still lacks sensitivity. Sensitivity of CA125 is about 80%, which means that 20% of patients suffering from ovarian cancer have CA125 levels within the normal range [43]. HE4 levels have been reported to be elevated in over a half of ovarian cancer patients with CA125 values not increased [44]. Despite this fact, overall sensitivity of HE4 is about 74% [43]. Sensitivity of Risk of Ovarian Malignancy Algorithm (ROMA), which is a diagnostic index combining CA125 and HE4 levels to evaluate the risk of ovarian cancer, is estimated at about 87% [43].
Another drawback of CA125 is its low sensitivity in detection of the early stage of ovarian cancer. Among other available biomarkers, HE4 has been reported to be the most sensitive (46.2% sensitivity at 90% specificity) [44]. Thus, efficacy of ROMA in diagnosis of early stage ovarian cancer is limited [45, 46]. Consequently, as far as diagnosis of early ovarian cancer is concerned, sensitivity of any biomarker or combination of markers seems to be rather unsatisfactory at the moment.
Moreover, neither CA125 nor HE4 is specific for all histopathological types of ovarian cancer. Although CA125 is overexpressed in most serous ovarian cancers, its levels remain within the normal range in about 70% of patients suffering from mucinous, clear-cell and endometrioid ovarian cancer [47]. HE4 is overexpressed in the vast majority of serous and endometrioid, over a half of clear-cell and very rarely in mucinous ovarian cancers [48, 49]. According to these data, both CA125 and HE4 are barely useful in diagnosis of less frequent types of ovarian cancer. Thus, a lot of investigators are still looking for new biomarkers and prognostic factors for ovarian cancer [2, 4, 24, 50, 51].
In conclusion, our results showed that CX3CL1 and CXCL1 are elevated in sera of ovarian cancer patients, which points to their role in cancer development. As we discussed, present diagnostic protocols of ovarian cancer need improvement regarding both sensitivity and specificity, which might be achieved by application of new biomarkers. Serum levels of CX3CL1 and CXCL1 might be useful in preoperative differential diagnosis of ovarian tumors, especially as they were not elevated in cases of endometriosis.