Introduction
Colorectal cancer is one of the most common human cancers, ranking third in terms of incidence and second in terms of mortality among all types of cancer. According to the 2018 GLOBOCAN report, the disease incidence is 19.7/100,000, and its associated mortality is 8.9/100,000 [1]. The standard of care in rectal cancer patients is surgical resection with total mesorectal excision complemented by radiotherapy and/or chemotherapy, depending on disease severity [2, 3].
Improvements in surgical techniques and better adjuvant treatment has led to a decrease in the number of abdominoperineal resections (APR) performed in favour of less invasive operations with GI tract continuity restoration. Such treatment offers higher five-year survival rates and improves some aspects of patient quality of life [4–6]. However, an increase in low anterior resections of the rectum is associated with a higher incidence of postsurgical bowel dysfunction, known as lower anterior resection syndrome (LARS) [7]. According to numerous authors, LARS consists of over 30 different symptoms [8], thus making it difficult to clearly categorise and predict which patients are at considerable risk of developing LARS after undergoing lower anterior resection due to rectal cancer. In 2012, a Danish study was published in which the authors suggested five main aspects of LARS and created a dedicated questionnaire to objectively assess its onset and severity in individual patients [9]. Those five aspects were flatus control, liquid stool leakage, frequency of bowel emptying, need to empty bowels within 1 h of the last bowel emptying, and urgency. Each aspect is given a numerical score based on the patients’ response, and the sum of all scores place patients in either a no LARS, minor LARS, or major LARS group. The questionnaire made the preoperative conversation easier and allowed more unified comparison of patients; however, it still did not offer any benefit in terms of preoperative assessment of the risk of developing LARS post-surgery. In 2018, POLARS was developed. This internet tool is a clinical survey that considers several key factors in patients undergoing lower anterior resection due to rectal cancer to assess the risk of developing LARS post-surgery. To calculate the score, the following data are required: gender, age at surgery, total/partial mesorectal excision, tumour height, preoperative radiotherapy, and stoma. The tool calculates a numerical score of 0–42, which places patients in one of three categories: no risk of LARS (0–20 points), minor risk of LARS (21–29 points), or major risk of LARS (30–42 points). This tool potentially offers the opportunity to provide more patient-tailored treatment (altering postoperative care in high-risk patients) and acquire more adequate informed consent with better patient understanding of the potential for bowel dysfunction after surgery [10].
The aim of our study was to assess how accurately POLARS can predict the development of LARS in patients undergoing lower anterior resection due to rectal cancer. We also aimed to determine the actual severity and frequency of LARS in patients undergoing this procedure at our centre.
Material and methods
In this study, we analysed all consecutive patients undergoing lower anterior resection due to rectal cancer at our centre during a 2-year period between January 1st, 2016 and December 31st, 2017. For each patient, peri-operative data, including age at surgery, gender, total mesorectum excision (TME)/partial mesorectum excision (PME), tumour height, preoperative radiotherapy, and stoma, were gathered. Each patient was then assessed with POLARS retrospectively and categorised into one of three groups according to the risk of developing LARS based on their POLARS score (no risk of LARS, mild risk of LARS, major risk of LARS). The postop treatment was not altered in any way based on the results of the POLARS prediction score because the assessment occurred retrospectively long after surgery. After an average 17 months post-surgery, a check-up was performed via telephone interview, during which the LARS questionnaire was filled out with the patient. The LARS numerical score and LARS category were documented. The actual LARS score was then compared with the predicted POLARS score in each of the patients, and the level of agreement and its statistical significance were assessed. Further descriptive analysis of all mispredicted cases was performed in search of a pattern.
Statistical analysis
Descriptive statistics were used for quantitative analysis. Mean and standard deviation statistics were used where appropriate. Categorical variables were compared using the χ2 test. The level of agreement between the POLARS and LARS scores and its statistical power was assessed using Cohen’s κ statistics. One-way ANOVA was used to compare characteristics between the three LARS groups.
Results
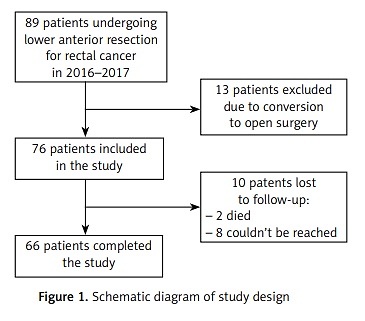
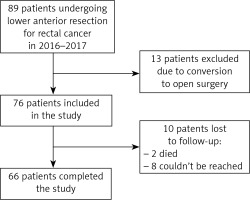
Between January 1st, 2016 and December 31st, 2017, 89 patients underwent laparoscopic lower anterior resection due to rectal cancer at our centre. Because of the need for conversion to an open procedure, 13 cases were excluded from the study. At the time of telephone check-up, 10 patients were lost to follow-up: 2 had died, and 8 could not be reached after numerous attempts. Thus, 66 patients were ultimately included in the study. The inclusion process is shown in Figure 1.
The mean age of the patients was 62.55 years (standard deviation (SD): 10.2 years; range: 37–81 years). The group consisted of 30 males (45.5%) and 36 females (54.5%). The mean POLARS score was 24.5 points (SD 4.06 points, range: 19–35 points), and the median was 24 points, which would place the ‘average patient’ in the risk of minor LARS category. The no-LARS category included 10 patients, the mild-LARS category 48 patients, and the major-LARS category 8 patients. The group characteristics and detailed POLARS score data are presented in Table I.
Table I
Group characteristics according to POLARS prediction categories
The mean follow-up time was 17 months (SD 8.6, range: 11–29 months). On telephone check-up, the LARS score was calculated for each patient. The mean LARS score was 16.42 points (SD 13.62, range: 0–39 points) and the median score was 15 points, which put the ‘average patient’ in the no-LARS category, which did not correlate with the POLARS prediction.
Based on the LARS questionnaire, 36/66 patients (54.5%) were in the no-LARS category, 18/66 (27.3%) were in the minor-LARS category, and 12/66 (18.2%) were in the major-LARS category.
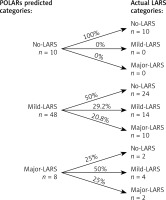
Interestingly, 10/10 (100%) patients who were predicted to be in the no-LARS category ended up in the same category after telephone check-up. Of 48 patients predicted to be in the mild-LARs category, only 14 (29.2%) ended up in this category, with 24 (50%) ending up in the no-LARS category and 10 (20.8%) ending up in the major-LARS category. Of 8 patients predicted to be in the major-LARS category, 2 (25%) stayed in the same category, 4 (50%) moved to the mild-LARS category, and 2 (25%) moved to the no-LARS category. A graphic representation of prediction vs. actual LARS score is depicted in Figure 2.
Overall, a correct prediction was noted in 26 (39.4%) cases, which was below expectations. Cohen’s κ statistic for the level of agreement was 0.130 (low level of agreement), p = 0.042. There were a total of 40 mispredicted cases (60.6%). Interestingly, upon analysis of the mispredicted cases, we found that 30/40 (75%) ended up in a lower LARS category than predicted, which means that on telephone check-up, patients expressed better bowel function than expected based on the POLARS tool assessment. Only 10/40 (25%) of mispredicted patients reported worse bowel function than that predicted by the POLARS tool. Those patients ended up in a higher LARS category at follow-up.
Discussion
Laparoscopic lower anterior resection is an increasingly common procedure to treat rectal cancer. Its obvious benefits include GI tract continuity restoration at the initial procedure, less invasiveness compared to APR, a better 5-year survival rate, and shorter recovery time after surgery [11]. However, lower anterior resection can lead to symptoms that negatively affect the quality of life. Those symptoms include urgency, faecal incontinence, frequent bowel movements, and difficulties in emptying the bowels [12]. This cluster of symptoms is referred to as LARS and is estimated to affect as many as 60–90% of all patients after lower anterior resection surgery [13].
The pathophysiology of this phenomenon is not fully understood. Surgical and radiotherapy-induced afferent visceral nerve damage could be the underlying cause [12]. A small study by Bregendahl et al. [14] showed that neoadjuvant radiotherapy in rectal cancer patients can cause neorectal hyposensitivity to both mechanical and thermal stimuli and can also lead to significantly lower anal resting pressure compared to patients who had not had radiotherapy before TME. The radiation-induced injury to the internal anal sphincter (which is more susceptible to ionising radiation than the external anal sphincter) may often lead to incontinence, because the internal anal sphincter is responsible for constant closure of the anal canal between bowel movements. In another study, Chen et al. [13] determined that major LARS was observed in 56% of patients who underwent TME with prior neoadjuvant radiotherapy compared to only 35% of those who did not have radiotherapy before TME surgery. The mean occurrence of major LARS in the study was 46% [13].
Another plausible hypothesis is a disturbance in colon and neorectal motility with enhanced postprandial response in patients after surgical rectal resection. Studies have shown a significant increase in neorectal pressure after a meal post-surgery in patients with major LARS compared to those with no LARS [15, 16]. The cause of this disturbance remains unidentified.
Neorectal reservoir dysfunction with reduced functional capacity may also play a role in LARS. Surgical denervation and a loss of stool-storing function of the rectum after resection are unavoidable. Reduced neorectal reservoir volume after end-to-end colorectal or coloanal anastomosis was believed to cause urgency and incontinence. A larger neorectal reservoir achieved with an altered surgical technique (colonic J pouch, coloplasty) showed benefits lasting only up to 12 months. Comparative studies analysing different techniques of anastomosis and creation of a larger reservoir volume of the neorectum have not shown any long-term benefits in terms of frequency and incontinence [17].
The treatment of LARS remains a challenge, and a multimodal approach should be implemented. Therapy is empirical and symptom based. In minor-LARS patients, medical management is advised; however, few high-quality data are available regarding this matter. Loperamide treatment for diarrhoea, 5-HT3 receptor antagonists for postprandial urgency and incontinence, and simethicone for gas and bloating remain the treatments of choice in this group of patients [18–21]. Dietary restrictions, high fibre consumption, and probiotics are advised, although no data support the benefit of those agents. For major LARS patients, additional procedures such as pelvic floor rehabilitation (in the form of biofeedback, electrostimulation, pelvic floor muscle training, and volumetric training) and transanal irrigation are available and of some benefit [22–24].
With LARS being a significant problem greatly affecting the quality of life of patients undergoing low anterior resection surgery for rectal cancer, it is our duty to thoroughly inform patients of the possibility of developing life-altering symptoms post-surgery. Because LARS is multimodal and the pathophysiology is still not fully understood, it remains difficult to predict which patients will develop LARS and to what extent after surgery. The POLARS tool for predicting LARS scores in patients undergoing this procedure offered a promising opportunity to better inform the patient about possible bowel dysfunction after surgery. However, our study has shown a low level of agreement between predicted and actual LARS scores. Only 39% of patients ended up in the same category as predicted by POLARS, and Cohen’s κ agreement score was as low as 0.130. This finding was disappointing, although further analysis showed that the POLARS tool is rather conservative, and 75% of all mispredicted cases showed better bowel function on follow-up than predicted by POLARS. Furthermore, 100% of the patients who were grouped in the no-LARS category by the POLARS tool reported no LARS on follow-up. Thus, the predictive value in this group of patients is strong and may be useful during pre-operative counselling.
In our study, 30/66 (45%) patients reported some sort of bowel dysfunction on follow-up (mild or major LARS) showing how common this disorder is. Major LARS was noted in 12/66 (18%) of our patients. This group of patients showed the highest negative impact of surgery on quality of life. Of those 12 patients, only two were grouped in the “major LARS” category by the POLARS tool, whereas the remaining 10 were in the “mild LARS” category by POLARS prediction. This outcome should be remembered by physicians when dealing with patients categorised in the mild-LARS group upon perioperative assessment using POLARS.
Mild LARS was observed in 27% of patients, and no LARS in 55%. This is not consistent with Batersby’s findings published in 2018 with the introduction of the POLARS tool. He recorded the prevalence of major LARS at 43%, mild LARS at 23%, and no LARS at 34%. These differences may be due to a different patient cohort or a higher number of patients with a shorter distance between the tumour and the anal sphincter in Batersby’s study compared to ours. It has been shown that the higher the tumour, the smaller the risk of developing LARS post-surgery. van Heinsbergen et al. [25] showed a significant difference in the prevalence of major LARS between sigmoid resection patients (20%) and colon resection patients (90%).
It is important to remember that LARS is not the only tool developed for the classification of postsurgical bowel dysfunction. Keane et al. [8] conducted a meta-analysis of all topic-related studies published between 1986 and 2006; it was determined that researchers have used as many as 18 different tools to assess bowel function post-surgery and its effect on the quality of life. Most of the tools only assessed stool incontinence, and in 36% of the studies no established assessment tool was used, but only a combination of symptoms was recorded [8].
Perhaps the incidence of LARS may be related to the genetics of the disease, which are not captured in this study. Currently many rectal cancer studies are taking place that are trying to define the exact aetiology of the disease. One of such studies is focusing on the genetics of the disease and is attempting to determine certain gene variants that might be a specific early marker for the disease [26] – perhaps if we learn to catch the disease before it progresses to stages difficult to treat, we will no longer have to deal with LARs syndrome post-operatively. Not taking into account the complex background of the disease (including the genetic aspect) might be considered as a limitation of this study. Another suggested limitation of the study is not taking into account the post-operative radiotherapy in rectal cancer patients; this might be a confounding factor because the POLARS tool only takes into account the pre-operative radiotherapy in this group of patients. However, in our study, no patients underwent post-operative radiotherapy, so this could not have confounded the data.
In conclusion, POLARS is a promising tool in preoperative counselling and for tailoring postoperative care in patients undergoing low anterior resection. It was not as effective as we had hoped, but the low agreement level may stem from the fact that the number of patients included in the study was limited. It is important to highlight that this tool seems to be very effective in the no-LARS group, whereas in other groups the predictability of the LARS syndrome remains low. Further research is needed to fully assess the value of POLARS and to give clinicians a better understanding of the information and consequences associated with POLARS scores.