Introduction
Gastric cancer remains the fourth most common cause of cancer-related death. Approximately 107,000 deaths are reported annually in Europe [1]. In 2018, 14,700 newly diagnosed cases were estimated in Germany and ~140,000 in the whole Europe [2]. As far as the USA is concerned, ~27,510 new cases were estimated in 2019 [3, 4]. In Japan, gastric cancer remains the most common type of cancer in males [4]. Despite a decrease in the incidence of gastric cancer worldwide, there is an increasing tendency towards tumors of the esophagogastric junction [1].
The etiology of gastric cancer is multifactorial and the main risk factors nowadays are male gender, Helicobacter pylori infection, atrophic gastritis, previous partial gastrectomy, Ménétrier’s disease and alcohol consumption, with the latter associated mainly with non-cardia cancers [4]. Tramacere et al. in a meta-analysis found no association between alcohol and the risk of esophageal and gastric cardia adenocarcinoma, even at higher consumption levels [5]. Very early and well-selected cancers can be treated endoscopically [1, 4, 6]. Surgical resection with extended lymph node dissection is the only curative treatment for advanced (≥ Stage IB) gastric cancers or even early cases with a significant risk of lymph node metastases.
Siewert type III carcinomas are considered true gastric cancers. In Siewert type II cancers additional distal esophageal resection with more extensive lymphadenectomy is needed to obtain an adequate oncological resection [1, 4, 6]. Since the introduction of D3 lymphadenectomy, there has been a lot of discussion concerning the results and the complication rates. Recommended treatment in Europe and the USA is a D2 dissection [1, 4, 6]. D3 dissection was introduced in the early 1990s in Japan. In Erlangen, it was adopted and developed until 1995 [7, 8]. The benefit of the extended lymph node dissection is still under discussion. In Japan and Korea, according to the latest guidelines of the Japanese Gastric Cancer Association (JGCA) and the Clinical Practice Guidelines for Gastric Cancer in Korea, respectively, D2 lymphadenectomy is standard and indicated for potentially curable T2-T4 tumors, as well as for cT1N+ tumors. D3 dissection shows survival benefits, but in some studies is directly associated with increased morbidity and mortality rates [1, 4, 6, 9, 10].
In Europe and in the U.SA. perioperative chemotherapy is recommended nowadays for patients with clinical stage ≥ IB. As regards carcinomas of the esophagogastric junction, perioperative chemotherapy or neoadjuvant chemoradiation is recommended for T3 and T4 and/or N+ M0 tumors [1, 4, 6]. On the other hand, in Asia, postoperative adjuvant chemotherapy is the standard treatment for locally advanced gastric carcinomas as well as for those of the esophagogastric junction [9, 11].
In this retrospective study, we present the long-term outcome in patients diagnosed with gastric carcinoma, including those with adenocarcinoma of the esophagogastric junction (Siewert type II and III), after surgical treatment in the Surgical Department of the University of Erlangen in Germany since 2001.
Material and methods
From 2001 until 2014, 120 patients with adenocarcinoma of the esophagogastric junction (Siewert type II/III) and 470 patients with gastric carcinoma of the stomach underwent surgical resection in the Surgical Department of the University of Erlangen and were included in this study. Patient data including symptoms, diagnosis, mortality, morbidity, follow-up, operation type perioperative and postoperative therapy were assessed using the database of the Tumor Center of the University of Erlangen and analyzed retrospectively.
The Kaplan-Meier method was used to calculate the 5-year rates of overall survival. The significance level of statistical hypothesis testing procedures was preset at p < 0.05. We used the statistical software package SPSS version 21 (IBM, Armonk, New York, USA) for all analyses.
At the end of the study (2016.01.01) 326 patients had died, 4 patients were lost to follow-up and 260 patients were still alive. The median follow-up time of all patients was 38 months (range: 0–181) and that of the surviving patients was 83 months (5–181).
Results
Classification was based on the TNM and Lauren classification systems (Union for International Cancer Control, UICC) [1, 12]. The type of preferred therapy was always according to the European guidelines and after discussion of these cases in the interdisciplinary tumor board conference of the certified and high-volume surgical department in Erlangen. Demographic and histopathological data are presented in Table I.
Table I
Demographic and histopathological data, n = 590, 2001–2014
| Location | Gastric carcinoma (%) | Siewert II carcinomas (%) | Siewert III carcinomas (%) |
|---|---|---|---|
| Gender: | |||
| Male | 280 (59.6) | 79 (84) | 21 (80.8) |
| Female | 190 (40.4) | 15 (16) | 5 (19.2) |
| Pathological UICC stage: | |||
| I: | 159 (33.8) | 14 (14.9) | 3 (11.5) |
| IA | 118 | 10 | 2 |
| IB | 41 | 4 | 1 |
| II: | 82 (17.4) | 7 (7.4) | 0 |
| IIA | 48 | 4 | 0 |
| IIB | 34 | 3 | 0 |
| III: | 84 (17.9) | 18 (19.1) | 6 (23.1) |
| IIIA | 26 | 10 | 3 |
| IIIB | 26 | 2 | 0 |
| IIIC | 32 | 6 | 3 |
| IV | 46 (9.8) | 3 (3.2) | 0 |
| X (not available) | 5 (1) | 6 (64) | 3 (11.5) |
| ypT0 ypN0 M0 | 3 (0.6) | 13 (13.8) | 1 (3.8) |
| yI: | 13 (2.8) | 3 (3.2) | 1 (3.8) |
| yIA | 9 | 2 | 1 |
| yIB | 4 | 1 | 0 |
| yII: | 21 (4.5) | 7 (7.4) | 3 (11.5) |
| yIIA | 15 | 5 | 1 |
| yIIB | 6 | 2 | 2 |
| yIII: | 21 (4.5) | 11 (11.7) | 5 (19.2) |
| yIIIA | 7 | 1 | 1 |
| yIIIB | 11 | 5 | 2 |
| yIIIC | 3 | 5 | 2 |
| yIV | 34 (7.2) | 11 (11.7) | 4 (15.4) |
| yX (not available) | 1 (0.2) | 1 | 0 |
| Total | 470 | 94 | 26 |
| Grading: | |||
| G1 | 29 (6.2) | 4 (4.2) | 0 |
| G2 | 104 (22.1) | 24 (25.5) | 4 (15.4) |
| G3 | 307 (65.3) | 51 (54.2) | 17 (65.4) |
| G4 | 6 (0.9) | 3 (3.2) | 0 |
| GX (not practicable) | 24 (5.1) | 12 (12.8) | 5 (19.2) |
| Lauren classification: | |||
| Intestinal | 223 (47.4) | 48 (51.1) | 14 (53.8) |
| Diffuse | 226 (48.1) | 30 (31.9) | 8 (30.8) |
| Unknown (not practicable) | 21 (4.5) | 16 (17) | 4 (15.4) |
| R classification: | |||
| R0 | 401 (85.3) | 86 (91.5) | 22 (84.6) |
| R1 | 21 (4.5) | 2 (2.1) | 1 (3.8) |
| R2: | 41 (8.7) | 4 (4.2) | 2 (7.7) |
| R2a* | 4 | 0 | 0 |
| R2b** | 26 | 4 | 2 |
| R2c*** | 11 | 0 | 0 |
| RX (not available) | 7 (1.5) | 2 (2.1) | 1 (3.8) |
| Total R0 | 509 (86.3) | ||
Ninety-three patients received neoadjuvant chemotherapy, while 59 were treated with neoadjuvant chemo-radiation. Adjuvant chemotherapy was administered in 98 patients, adjuvant chemo-radiation in 8 patients. Thirty-one patients with gastric cancer and one with Siewert II adenocarcinoma were treated with palliative chemotherapy, while 4 patients of the gastric cancer group received palliative chemo-radiation. Data are presented in further detail in Table II.
Table II
Perioperative treatment
Our D3 LND technique was not completely identical to that proposed by the Japanese Research Society for Gastric Cancer, as described in detail elsewhere [7]. Differences were restricted to the further subdivision of lymph node stage 16 (16aoR, 16auR, 16b1R and 16aL) in compartment D3. These stations were evaluated separately as specimens in order to evaluate, eventually in future studies, the role of each “sub-compartment” in the long-term oncological outcomes.
Lymph nodes of compartment D1, including stage 7 (left gastric artery) in compartment D2, were removed en bloc with the resected gastric specimen. All other lymph nodes were defined by the surgeon and dissected individually during surgery [7, 8]. We did not perform any prophylactic pancreatectomy or splenectomy. Splenectomy was performed in 76 patients in cases of spleen or hilum involvement. The preferred types of operation and the methods of reconstruction are presented in Table III.
Table III
Type of operation and reconstruction, n (%)
Concerning the gastric carcinomas, the median oral resection margin was 5.5 cm (0–19 cm), while it was 2 cm (0–14 cm) for the Siewert II and 2.5 cm (0.1–13.7 cm) for the Siewert III adenocarcinomas. Distally, a minimum of 3 cm of duodenum was always resected for an adequate gastrectomy. R0 resection was achieved in 86.3% of all cases; 87.9% of patients underwent primary surgery and 81.7% of those with neoadjuvant therapy, while the difference was marginally significant (p = 0.056).
Fifteen (2.5%) patients had D0 resection, mostly for palliation, while 66 (11.1%), who were not medically fit for extensive nodal dissection, had D1 resection. D2 resection was performed in 46.3% of cases, while lymph nodes in the splenic hilum were included in 48 patients. 237 (40.2%) patients, who had D2 with interaortocaval lymph node dissection, were classified as D3. Although D1+ is an accepted technique, especially for early gastric cancers, is not adopted in the Surgical Department of Erlangen, as it is not yet routinely recommended according to the European, German and N.C.C.N. guidelines [1, 4, 6].
The median number of harvested lymph nodes was 33 (2–89) for patients diagnosed with gastric cancer and 29 for those with adenocarcinomas of the esophagogastric junction (5–97 for Siewert II and 10-79 for Siewert III carcinomas). There was no statistical significance in patients who underwent neoadjuvant therapy. In detail, median examined lymph nodes after neoadjuvant therapy vs. primary surgery: 28 (3–72) vs. 34 (2–97): p = 0.002 for every stage and localization.
Postoperative complications were classified according to the scale of Clavien-Dindo [13]. Concerning surgical complications, the overall anastomotic leak rate was 3.6%; 3.2% in patients with gastric cancer and 5% in those with adenocarcinoma of the esophagogastric junction. A pancreatic fistula developed in 10 (1.7%) cases. Twenty-seven (4.6%) patients were re-operated on while an interventional procedure was preferred in 50 (8.5%) patients. Early in-hospital mortality was 5.4%; 2.2% of the patient deaths in the early postoperative period were due to non-surgical complications while 3.2% were due to surgical ones (Table IV). Morbidity and mortality were not significantly different in patients who received chemo- and/or radiotherapy compared to those who underwent primary surgery (Table V).
Table IV
Postoperative complications according to the scale of Clavien-Dindo*
Table V
Postoperative morbidity and in-hospital mortality
| Localization | Neoadjuvant therapy | Primary surgery |
|---|---|---|
| Morbidity* (%): | ||
| Stomach | 29.5 | 26.9 |
| p = 0.620 | ||
| Siewert II | 34.8 | 41.7 |
| p = 0.492 | ||
| Siewert III | 28.6 | 50 |
| p = 0.422 | ||
| Total | 31 | 29.2 |
| p = 0.678 | ||
| In-hospital mortality (%): | ||
| Stomach | 4.2 | 4 |
| p = 1.0 | ||
| Siewert II | 13 | 8.3 |
| p = 0.519 | ||
| Siewert III | 14.3 | 8.3 |
| p = 1.0 | ||
| Total | 7.7 | 4.6 |
| p = 0.138 | ||
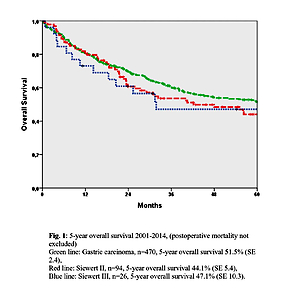
The 5-year overall survival rate was 51.5% for gastric carcinoma, 44.1% for Siewert type II and 47.1% for Siewert III cancers of the esophagogastric junction, including the mortality (Figure 1).
Figure 1
5-year overall survival 2001–2014 (postoperative mortality not excluded). Green line: gastric carcinoma, n = 470, 5-year overall survival 51.5% (SE 2.4), red line: Siewert II, n = 94, 5-year overall survival 44.1% (SE 5.4), blue line: Siewert III, n = 26, 5-year overall survival 47.1% (SE 10.3)
Discussion
When our long-term results are compared with others, it is striking that there are both similarities and differences. Approximately two thirds (64.4%) of the 590 patients with gastric carcinoma or adenocarcinoma of the esophagogastric junction treated in the last 14 years were male, which was also confirmed in other studies [4]. A very high percentage (44.2%) of our study group was of advanced stage disease (p- or yp-III and IV).
Many studies have confirmed increasing use of perioperative forms of therapy, due to better survival rates [14–21]. The percentage of our patients who received neoadjuvant chemotherapy or chemoradiation or perioperative chemotherapy (25.8%) seems to be low but the number increased remarkably, especially in the last few years. At the beginning of the study, chemotherapy and radio-chemotherapy were rarely used in Germany. Gradually, these therapies became more widely available as treatment options improved. It was similar in our clinic. Moreover, about 50% of the carcinomas included in this study were of diffuse type, which – in our opinion – is not quite “chemo-sensitive”. Concerning the positive effect of neoadjuvant or perioperative chemo- or radio-chemotherapy, we should point out that 74.2% of the patients had no perioperative therapy. Moreover, the number of patients with stage II or III disease who received combined treatment was almost half the number of those who underwent primary surgery (103 vs. 196). Therefore, the comparability of these two groups is limited and possibly biased.
Regarding the operation, there has also been an obvious trend to a safety margin of 5 and 8 cm respectively according to the Lauren classification (intestinal/diffuse). As a result, either a 4/5 resection or gastrectomy is carried out then. Three forms of reconstruction are still widely employed: Billroth I, Billroth II and Roux-en-Y with resection, and Roux-en-Y and occasionally Billroth II with gastrectomy. Our preferred method with aboral resection is Billroth I reconstruction, as this is the most physiological one, in our opinion, even if the risk of postoperative anastomotic leak is higher in the event of iatrogenic injury of the pancreas due to the dissection of the lymph nodes around the pancreatic head. Our operated cancers, reconstructed with BI, were also of advanced stages. Attention should be paid in order to achieve intraoperatively adequate mobilization of the duodenum without damaging the pancreatic head, in order to avoid any anastomotic leak or pancreatic fistula. We were able to do it whenever an adequate oral safety distance was available. Exceptions were, of course, tumors that infiltrated adjacent structures. The less extensive safety margin achieved in Siewert II–III could possibly explain the worse outcome of cardia cancer, as the median number of harvested nodes is approximately the same. Possibly the anatomy of this area and/or the molecular behavior of the tumors located at the esophagogastric junction also play an important role, but it should be evaluated in further detail.
Other reconstructions predominated in approximately 60% of the gastrectomies. We preferred Roux-en-Y reconstruction as we observed prolonged convalescence and an increased risk of postoperative gastrointestinal transit disorders with other operation techniques, especially Billroth II. Ultimately, however, when comparing literature, it remains unclear which method has a better outcome. Some studies have found advantages in Roux-en-Y reconstruction, but this was not confirmed in other studies. The method learned by the surgeon and the associated operative experience undoubtedly play a major role [8, 10, 13, 22–30].
The current UICC TNM classification recommends excision of at least 15 lymph nodes for accurate staging [1, 4, 12]. Contrary to the general opinion in the literature, where D2 dissection is regarded as adequate, we performed D3 dissection in approximately 40% of the cases. This is reflected by the number of lymph nodes we removed. The median number of harvested lymph nodes was 33 (2–89) for patients diagnosed with gastric cancer and 29 for Siewert II (5–97) and III (10–79) carcinomas, while removal of 25 lymph nodes is required for complete D2 dissection according to the German guidelines. It should be mentioned that even though a dissection was strictly classified as D2, the number of harvested nodes was greater than required for an adequate D2 dissection.
Lymph node stations 13–15 were removed routinely during D3 dissection. Complete interaortocaval dissection of lymph node station 16 was not always performed. It was omitted when the tumor was located proximally and the patient was of advanced age and increased comorbidity. However, it was performed with curative intent in the case of distally located diffuse cancers. The pre-esophageal lymph nodes (LN station 116) were removed routinely with tumors of the esophagogastric junction. As mentioned above, since 2001, 237 patients (40.2%), who had D2 resection with partial or complete interaortocaval lymph node dissection, were classified as D3. We officially included the term D3 gastrectomy in our tumor documentation until 2010. Indeed, from 2010 to 2014, 56 patients (43.4%) had D3 dissection, showing that the D3 dissection rates were similar to those of 2001–2010. Under this aspect, the term “interaortocaval lymph node dissection” used to identify patients with D3 dissection in the early 2000s can be regarded as completely reliable.
Despite the extended lymph node dissections, our postoperative complication rates were acceptable. The rate of anastomotic leak was as low as 3.6%; 3.2% in patients with gastric cancer and 5% in those with adenocarcinoma of the esophagogastric junction. The incidence of anastomotic leakage was relatively low compared with reported data showing that this life-threatening complication could vary between 6.9 and 12.3% in cases of esophago-intestinal anastomosis. In a retrospective analysis of 1114 patients who underwent a total gastrectomy for gastric cancer in the University of Hannover in Germany, the overall anastomotic leak rate was 7.5% [31]. Pancreatic fistulas occurred in 1.7% of the patients and were assessed clinically by the drainage secretion.
Overall in-hospital mortality was 5.4%, including both true gastric carcinomas and adenocarcinomas of the esophagogastric junction and 3.2% of the patient deaths in the early postoperative period were due to surgical complications. These results suggest decreased short-time mortality compared to the mean of high-volume centers in Germany, which have been reported to be as high as 5.8% [32]. In a retrospective study of Booka et al. performed on 284 patients with esophageal cancer, the in-hospital mortality was 2.1% and the 30-day mortality 0.7%. On the other hand, the anastomotic leak rate was as high as 19.4% while the overall surgical complication rate was 40.1% [33]. Kawaguchi et al. in 2016 reported a total of 20% for Clavien ≥ 2 complications after curative gastrectomy (R0 or R1) with lymph node dissection, while in our study this rate was 22.4% [34]. Moreover, morbidity and mortality were not significantly increased in patients who received combined treatment compared with those who underwent primary surgery.
The decreased complication rate in Asia after gastrectomy could be associated with the average body mass index (BMI). In a previous comparison study in patients with colonic cancer in Erlangen and in Japan, BMI was significantly higher in Erlangen compared with Japanese patients (median, 26 vs. 22 kg/m2) [35]. In the future another study analogous to this one could be useful in order to evaluate BMI as a potential independent factor affecting the postoperative morbidity and in-hospital mortality rate in this patient group.
A recent study demonstrated that hospitals with high annual volume (≥ 21 operations/year) were associated with increased survival rates [36]. According to the SEER database, the 5-year overall survival rate for patients diagnosed with gastric cancer in the USA is 31.5% for every stage [3]. We achieved a 5-year overall survival rate of 51.5% (SE 2.4%) for gastric carcinoma, 44.1% (SE 5.4%) for Siewert type II and 47.1% (SE 10.3%) for Siewert III cancers of the esophagogastric junction. Multimodal and personalized treatment models, combined with extended lymph node dissections, resulted in prolonged average 5-year survival compared with other patient populations, while the complication rate was acceptable [27–31, 34, 36–41]. It would be of great interest to evaluate comparatively our patient data from 2015 until 2020, as in the last decade perioperative multimodal treatment concepts have finally become established.
In conclusion, the extent of lymph node dissection remains controversial, as it may increase the perioperative complication rate. Nevertheless, we achieved better 5-year survival in our patients with modified D3 dissection compared with other reference groups of patients, while the complication rate was acceptable, even in patients who underwent combined treatment.