Introduction
Diabetes mellitus (T2D) is a metabolic disorder increasing in prevalence with elevated morbidity and mortality globally [1, 2]. It is estimated that the number of T2D patients will increase from 382 million in 2013 to 592 million by 2035 [3]. Diabetes has several complications, such as nephropathy, retinopathy, peripheral neuropathy, and cardiovascular diseases, which are associated with increased hospitalization rates and mortality [4, 5]. Trehalose is a naturally occurring disaccharide of 2 glucose molecules approved by the Food and Drug Administration [6]. Trehalose is found in many organisms and plants as an energy source and comprises 2 D-glucose links by the reducing end α-1,1 in a glycosidic bond, which is more resistant to acid and α-glycosidase cleavage [7]. Trehalose has been used as a sweetener and an additive in the food industry, a moisturizing agent in cosmetic products, and a stabilizer in pharmaceutical formulations such as antibodies, enzymes, liposomes, and genomic material [8, 9]. Recent reports have stated that trehalose evokes a lower glycaemic response and reduces insulin resistance compared to other disaccharides [10–12]. In addition, there is evidence for its antioxidant [13], anti-inflammatory [14, 15], and autophagy-enhancing properties [16, 17] with therapeutic potential for several common health problems, including cardiometabolic disorders [18–21] and neurodegenerative diseases [22–26]. Becuase T2D is associated with inflammation pathways, endoplasmic reticulum (ER) stress and oxidative stress, and impairment in the autophagic process [27, 28], trehalose may decrease diabetes-related health problems. In healthy subjects, oral trehalose ingestion resulted in lower blood glucose, insulin levels, as well as lower active gastric inhibitory peptide (GIP) levels compared to glucose ingestion [29]. Others reported that daily consumption of 3.3 g of trehalose improved glucose tolerance in nondiabetic individuals with higher postprandial glucose levels [30]. Moreover, clinical studies in overweight people showed reduced glycaemic and insulinaemic responses following trehalose intake, which may improve metabolic syndrome [12, 31–33]. However, prospective randomized controlled trials on trehalose therapy in T2D are few; therefore, this clinical research was undertaken.
Material and methods
Study design
Forty participants aged 35–85 years with T2D, who were referred to the endocrinology and metabolism clinic from January to May 2022 were selected for enrolment in the present study, which was designed as a parallel, double-blind, randomized, placebo-controlled clinical trial. The protocol was registered at the Iranian Registry of Clinical Trials with the number IRCT20130829014521N18 (https://en.irct.ir/trial/54391) and approved by the Institutional Ethics Committee and Research Advisory Committee of the National Institute for Medical Research Development (IR.NIMAD.REC.1399.267). All participants provided written informed consent. Inclusion criteria: fasting blood glucose ≥ 126 mg/dl and T2D with no change in medication over the preceding 3 months. Exclusion criteria: type 1 diabetes, insulin therapy, renal dysfunction (eGFR less than 30 ml/min), severe liver dysfunction or cirrhosis, usage of α-glucosidase inhibitors such as acarbose, oral or injectable corticosteroids usage, pregnancy, lactation or nursing, or wishing to conceive. If any individual was unwilling to continue the trial or had poor compliance with the trial treatment (< 80%), he/she was withdrawn from the study during the follow-up period.
Randomization and blinding
Eligible patients were randomized in a ratio of 1 : 1 to either the trehalose or the sucrose group, using a table of random numbers in permuted blocks of 4. A pharmacist provided a random list and coded treatment/placebo boxes into A and B before the study’s initiation, while other investigators, patients, laboratory staff, and outcome assessors were blinded to the treatment assignment until final analyses. Trehalose and sucrose were similar in terms of colour and odour. We used consecutively numbered, opaque, sealed envelopes to conceal the allocation sequence.
Intervention
Patients in the treatment and placebo groups received a box containing about 280 g trehalose and sucrose, respectively, and they were asked to consume 3.3 g/day (less than two-thirds of a teaspoon) after breakfast for 12 weeks. All patients were instructed to continue diabetes drugs as prescribed with no lifestyle changes.
Outcomes measures
Sociodemographic information was obtained baseline using a general questionnaire. Weight and height were measured at baseline using a digital score (Seca 831, Hamburg, Germany) to the nearest 0.1 kg with minimum clothing. Height was determined without shoes to the nearest 0.1 cm by a stadiometer (Seca 206, Hamburg, Germany). Physical activity was recorded at the beginning and end of the study using 24-hour recall, in which participants were asked to remember the duration (minutes) and intensity of their last 24 h of physical activities.
Serum biomarkers assessment
Blood samples (5 ml) were taken early in the morning after overnight (8 h) fasting at baseline and post-intervention in tubes containing a serum gel separator, and then they were centrifuged at 750 g for 15 min to obtain serum. Sera were aliquoted and were stored at –80°C until batch analysis. Glycated haemoglobin (HbA1c) was measured by ion-exchange chromatography. Serum insulin levels were determined using the (enzyme-linked immunosorbent assay) ELISA kit (Pars Azmoun kit, Tehran, Iran), and FBG was measured by the colorimetric technique (Pars Azmoun kit, Tehran, Iran). Moreover, the serum C-reactive protein (CRP) level was measured utilizing an immunoturbidimetric method (Bionik Diagnostic System, Tehran, Iran) and reagent kit (Pars Azmoun kit, Tehran, Iran). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: [(FPI (lU/ml) × FPG (mmol/l))/22.5] [34].
Pro-oxidant Antioxidant Balance (PAB) Assay
To evaluate whether trehalose could improve the antioxidant status, an investigation of (anti)oxidant parameters pre- and post-treatment was performed using commercial kits (Kiazist; Iran). In this investigation, PAB (pro-oxidant antioxidant balance) was measured to estimate the total oxidants and antioxidants in a single measurement as previously described [35, 36], which is based on the oxidation of the chromogen 3,3’,5,5’-tetramethylbenzidine (TMB) to a colour cation by pro-oxidants in an enzymatic reaction and reduction of the TMB cation to a colourless substance.
Psychological stability
To determine the therapeutic efficacy of trehalose consumption on psychological stability such as depression, anxiety, and stress, the DASS-21 scale was utilized at baseline and at the end of the interventional trial (Day 0 and week 12). DASS-21 is a test questionnaire involving 21 questions in 3 subscales (depression, stress, and anxiety). Each part contains 7 questions scored from zero (does not apply to me at all) to 3 (absolutely applies to me) [37]. Scores of the 3 subscales are calculated by summing the relevant responses and multiplying by 2. The minimum and maximum scores for each subscale are zero and 21 (if multiplied by 2, it is between zero and 42), and a lower score indicates less anxiety, depression, and stress. The validity and reliability of the scale have been previously confirmed in Iran [38].
Quality of life
The diabetes quality of life (DQOL) index was assessed using a treatment-focused version of the DQOL scale [39]. This index involves 15 items; each question is scored from one (very dissatisfied) to 5 (very satisfied). The minimum and maximum scores for each subscale are 15 and 75. A higher score indicates a better QOL. The validity and reliability of this scale have been previously confirmed in Iran [40].
Statistical analysis
Statistical analyses were performed with SPSS Statistics, Version 25 (IBM). The sample size was calculated considering a type I error of 0.05 and power of 80% to detect a 0.5-unit change in HOMA-IR and an interpersonal variation of 0.5. It was estimated that 17 subjects would be needed in each group. Considering a dropout rate of 15%, a total of 20 subjects were considered in each group. The distribution of quantitative variables was checked, then Student’s t-test and the Mann-Whitney U-test were used to compare means of data between 2 groups. The χ2 test was used to compare the qualitative variables, and ANCOVA analysis was used to adjust the results where significant differences in baseline values were present. Results with p < 0.05 were considered as statistically significant.
Results
Clinical characteristics of patients
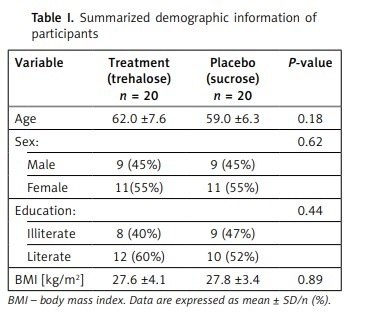
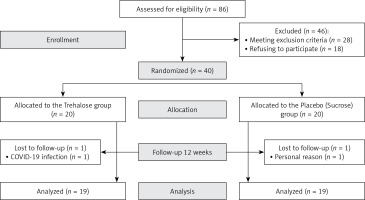
A total of 86 patients were assessed for inclusion in the study, of whom 40 met the inclusion criteria and were randomized into 2 groups. Thirty-eight subjects completed the trial (intervention group, n = 19; control group, n = 19, Figure 1, 2). One person in the trehalose group withdrew due to COVID-19 infection, and one subject in the control group withdrew from the supplementation due to undefined personal reasons. No unfavourable adverse effects were reported for either group. No significant differences were seen in demographic characteristics between groups (Table I).
Glycaemic indices levels
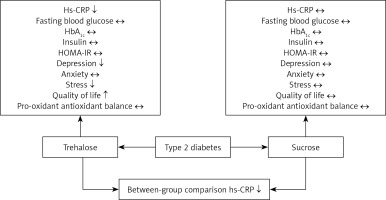
The glycaemic index analysis showed baseline differences in FBS and HbA1c levels between the treatment and placebo groups; patients assigned to the trehalose group showed significantly higher levels of FBS and HbA1c than those in the sucrose group (FBS, p = 0.02 and HbA1c, p = 0.04, respectively); however, a comparison of change values did not reveal any alteration in these parameters following trehalose consumption (p = 0.15 and 0.73 for FBS and HbA1c, respectively). The ANCOVA test did not suggest any difference in these values between the groups after adjustment for baseline values (p = 0.51 and 0.96 for FBS and HbA1c, respectively). There were no differences in the glycaemic indices of insulin and HOMA-IR between the treatment and placebo groups (Table II). However, HS-CRP differed between groups (p = 0.02). Analysis of PAB values showed no difference between trehalose and sucrose groups (p = 0.72) (Table II).
Table II
The effect of trehalose compared to placebo (sucrose) in a total of 40 patients on glycaemic indices at baseline and at the end of the study between groups
Mood status, quality of life, and physical activity
There was no difference in the depression, anxiety, and stress status of the patients at baseline. The depression and stress scores of the treatment group were significantly lower than those of the placebo group (p = 0.02 and p = 0.05, respectively) at the end of the study; however, the mean difference for mood status did not differ between treatments.
The quality of life score in the treatment group was higher than in the placebo group (p = 0.03); however, this difference was lost after the mean difference analysis.
The group time and intensity of physical activity did not differ between groups. The results of the intervention on the mood status, quality of life, and physical activity of the patients are shown in Table III.
Table III
The effect of trehalose on mood status, quality of life, and physical activity at W0 and W12
Discussion
This is the first randomized dietary intervention trial to assess the anti-hyperglycaemic effects of trehalose in patients with type 2 diabetes. It showed that trehalose decreases CRP as a mediator of inflammation and that there was an indication of overall quality of life being improved by trehalose supplementation. Whilst fasting blood glucose, insulin, insulin resistance, and HbA1c did not differ over the 12-week period, the change in CRP may, over a longer period, affect glycaemic control and potentially diabetes-related complications. Studies have shown that trehalose can modulate glucose metabolism and insulin sensitivity in healthy subjects [30–32]. Previous research has shown that 12-week trehalose ingestion improved glucose tolerance in subjects with BMI ≥ 23 kg/m2 and with a high risk for type 2 diabetes. Trehalose markedly reduced blood glucose after a 2-h OGTT following 3 months of intake in comparison with baseline compared to a sucrose group [19]. This suggests that one mode of action of trehalose would be to modulate the absorption of sugars to give a flat absorption process, thus avoiding marked glucose excursions; hence, while mean glycaemic control may not be affected, glucose variability may be reduced and needs further investigation. These studies have been supported by animal work showing that the administration of trehalose could effectively ameliorate the increased FBG levels, glucose intolerance, and insulin response in streptozotocin-induced diabetic rats [41].
Oral absorption of trehalose is decreased to 0.5% due to enzymatic metabolization by the trehalose enzyme in the intestinal brush border; hence, there are reports suggesting that intravenous trehalose is more effective for clinical trials [7, 8], but it is not convenient for routine clinical practice.
It has been reported that there are increased plasma levels of several biomarkers of inflammation, such as C-reactive protein (CRP), in patients with T2D [42]. A cross-sectional study of 120 subjects showed the mean hs-CRP level was 16.5 ±12.7 mg/l in diabetic patients, while the hs-CRP level was lower than 6.0 ±0.0 mg/l in those without diabetes [43]. The current study indicates that trehalose intake for 12 weeks could significantly reduce hs-CRP levels in diabetic patients. This finding could point to the potential use of trehalose for preventing incident atherosclerotic cardiovascular disease in patients with diabetes because inflammation is a key mechanism in atherosclerosis [44] and diabetes is itself a major risk factor for cardiovascular disease [45, 46].
Because oxidative stress is an essential pathogenetic factor for diabetes-related complications, an antioxidant therapy approach may be a helpful to augment diabetes treatment by preventing free radical production, boosting intracellular antioxidant defences, and providing protective mechanisms against oxidative stress-induced apoptosis and conserving β-cell function [47–49]. The antioxidant effects of trehalose have been evaluated in different in vitro and in vivo studies [50, 51]. This unique molecule can decrease the levels of reactive oxygen species and hydrogen peroxide levels in a dose-dependent manner in animal models of T2D [52, 53]. Furthermore, trehalose can upregulate antioxidant gene expression, such as superoxide dismutase, glutathione, and catalase, by promoting the nuclear translocation of Nrf2 [53–55]. However, in this study no difference in the oxidative stress index measure PAB was seen in the trehalose group compared to the placebo group.
The analysis of the DQOL questionnaire data suggest that trehalose treatment might increase health-related quality of life among diabetes patients, and the quality-of-life score in the treatment group was statistically higher than in the placebo group. Moreover, depression and stress scores in the treatment group were significantly lower than in the placebo group following 3 months of treatment with oral trehalose; however, these differences were not statistically significant after the mean difference analysis, which probably reflects the small number of patients for quality-of-life evaluation. Depression is associated with negative health consequences in diabetic patients [56–59] and diabetes-related complications lead to reduced quality of life and depression [60]; therefore, a larger study confirming the beneficial effects of trehalose on quality of life would be of major benefit in diabetes. Assessing health-related quality of life by a visual analogue scale (VAS) might be useful in evaluating the overall health of diabetic patients in the clinical setting [61].
Because this was a randomized and blinded trial the groups were allocated at random at baseline, and the baseline differences seen for fasting blood glucose, HbA1c, and HOMA-IR were due to chance alone; however, these differences in baseline values were taken into account using the ANCOVA analysis.
Our study has several limitations. A larger sample size to confirm the improvement in quality of life is needed because this study was powered for a change of a biochemical parameter. Given the potential mechanism of action of trehalose in diabetes, a clinical trial of longer duration with trehalose is needed.
In conclusion, 12 weeks of treatment with 3.3 g/day of oral trehalose significantly improves CRP as a marker of inflammation, with potential favourable effects on quality of life, depression, and stress levels, but overall glycaemic control and pro-oxidant antioxidant balance were unaltered over this time frame.