Glucose-6-phosphatase deficiency type Ib (GSD Ib) is a rare (OrphaNet classifier ORPHA: 79259) autosomal recessive glycogen storage disease and manifests as hypoglycemia with other metabolic disturbances such as lactic acidosis, hyperuricemia, and hypertriglyceridemia [1].
Neutropenia and neutrophil dysfunction resulting in recurrent infections, perioral and perianal infections, and abscesses are the hallmark features of GSD Ib [2]. The administration of granulocyte colony-stimulating factor (G-CSF) improves the neutrophil count but not dysfunction and increases the risk of myelodysplasia and acute myeloid leukemia [1].
There is no treatment for GSD Ib. To prevent metabolic disturbances and neurological deficits, a diet based on uncooked cornstarch given every 4 h is recommended [3].
In recent years it was shown that empagliflozin, sodium-glucose co-transporter type 2 inhibitor (SGLT2), reduces the risk of cardiovascular death in subjects with diabetes mellitus [4]. As a result, empagliflozin is recommended for the prevention of cardiovascular events in diabetic patients, regardless of the presence of cardiovascular disease [5]. Administration of the drug is also associated with a reduction in hospitalizations plus cardiovascular deaths in people with heart failure [6]. The main mechanism of empagliflozin action is associated with glucosuria due to inhibition of glucose re-absorption. This mechanism appeared to preclude the use of the drug in patients with GSD Ib. However, according to a study performed on rodents and several case reports of empagliflozin treatment, administration of empagliflozin, an SGLT-2 inhibitor, was beneficial in GSD Ib as it accelerated elimination of 1,5-anhydroglucitol-6-phosphate (1,5AG6P), which is responsible for neutrophil dysfunction [7–9]. 1,5AG6P is a structural analogue of glucose-6-phosphate and a product from phosphorylation of 1,5-anhydroglucitol physiologically present in the blood.
Here, we present the longest reported observation of 2 subjects with an advanced stage of GSD Ib treated with empagliflozin.
Methods
After diagnosis of GSD Ib the patients were under the care of the Children’s Memorial Health Institute in Poland. At the age of 26 and 25 years both brothers were referred to the Department of Internal Medicine, Hypertension and Vascular Diseases at the Medical University of Warsaw to continue medical care. Follow-up visits included clinical evaluation, blood tests, and imaging tests (including magnetic resonance of the liver) each half year. Shortly after the promising results of empagliflozin treatment in subjects with GSD Ib were published [7, 8], we offered the brothers an experimental treatment with this drug. Written consent for the off-label administration of empagliflozin was obtained and the protocol was accepted by the Bioethics Committee at the Medical University of Warsaw (KB/131/2020 and KB/132/2020, separately for each patient).
During out-patient follow-up visits, the patients’ clinical statuses, and tolerances of treatment were evaluated. The research team was available for 24 h a day, 7 days a week to offer medical advice and report possible adverse events. There was a series of scheduled visits for regular assessment and collection of blood and urine samples to repeat initial measurements. Due to the COVID-19 pandemic it was not possible to follow the planned experimental protocol with a prespecified time of visits, and some of them were performed remotely.
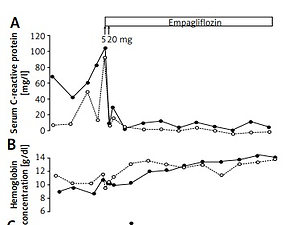
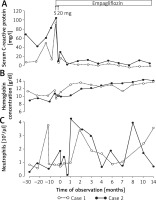
G-CSF was discontinued the day before empagliflozin was started. Empagliflozin was initiated in both patients at the same time (July 2020) at a dose of 5 mg/day and increased the next day to 10 mg/day. From the 8th day onwards, 20 mg/day was administered. Based on the neutrophil counts, which decreased after G-CSF withdrawal despite empagliflozin treatment (Figure 1), G-CSF was started in the lower dose again in both patients.
Results
Patient No. 1. Course of the disease before treatment with empagliflozin
He was diagnosed with GSD Ib at 6 months of age based on the results of liver biopsy, which was performed due to hypoglycemic episodes. During childhood, he underwent repeated esophageal dilatations because of recurrent strictures. He was diagnosed with inflammatory bowel disease (IBD) and treated with mesalazine (2015) and budesonide (2018), resulting in modest clinical improvement. The patient still reported episodes of abdominal pain with 6–7 watery stools per day and relapsing aphthous oral lesions.
In childhood lower respiratory tract infections were frequent. In 2016, he was diagnosed with nonallergic asthma. The patient received treatment with salmeterol and fluticasone propionate.
Since the age of 10 years, the patient has been receiving G-CSF 0.3 mg per day for neutropenia and recurrent infections. Despite the treatment, in 2017 the white cell number was decreased (3.1 K/µl) with the absolute neutrophil count (ANC) 0.89 K/µl. Aphthous gingivostomatitis and furunculosis recurred several times per year. Anemia was present with hemoglobin concentration 9.2 g/dl and mean cell volume 90 fl. Thrombocyte count was within the normal range. In July 2020, before empagliflozin treatment, the white cell count was 2.48 K/µl with ANC 0.46 K/µl. Hemoglobin concentration was 9.9 g/dl.
In 2017, he was diagnosed with heart failure with mid-range ejection fraction (ejection fraction 46%), hypertension, and sinus tachycardia. He was treated with metoprolol succinate, ramipril, and eplerenone, resulting in resolution of heart failure symptoms, good blood pressure control, and normalization of left ventricular ejection fraction. In July 2018 the NT-proBNP level was 184 pg/ml and left ventricle ejection fraction was 55%. It remained stable until June 2020 with the NT-proBNP level 69 pg/ml just before starting empagliflozin.
In 2017, glomerular hyperfiltration with albuminuria (albumin to creatine ratio 78.6 mg/g) and nephromegaly (L = 14 cm, R = 14 cm) were reported, and levels of triglycerides (264 mg/dl), uric acid (11.6 mg/dl), lactate (2.4 mmol/l), CRP (4.5 mg/dl), and γ-globulins were elevated. Before starting the experiment, in July 2020 triglycerides were 94 mg/dl, uric acid 9.6 mg/dl. Lactate concentration was elevated at 5.6 mmol/l. Serum total protein was increased (7.3 g/dl) with normoalbuminemia. CRP was 90.5 mg/dl.
Course of the disease after empagliflozin administration
In July 2020, when the patient was 29 years old, empagliflozin was started. By this time, his height and weight were 182 cm and 72 kg (BMI 21.7 kg/m2), respectively. During 12 months of empagliflozin treatment, he lost 4 kg despite unchanged dietary habits.
During the first few weeks after empagliflozin initiation his neutrophil count started to increase and allowed for dose reduction of G-CSF to 0.3 mg twice a week. Hemoglobin level also increased (Figure 1). After empagliflozin administration, CRP levels normalized immediately (Figure 1).
Only one episode of abdominal pain and two episodes of self-limiting oral lesions occurred during 12 months of empagliflozin treatment. The patient reported 2-3 well-formed stools per day. Hepatosplenomegaly persisted. One self-limiting upper respiratory tract infection occurred. During infection ANC was 9 K/µl. After receiving the second dose of BNT162b2 mRNA COVID vaccine, he had mild asthma exacerbation that resolved spontaneously.
During empagliflozin treatment there were no signs or symptoms of heart failure. Echo-cardiography revealed no abnormalities. Eplerenone was withdrawn, and the doses of metoprolol and ramipril were reduced. De-escalation in heart failure treatment has not resulted in a recurrence of resting tachycardia or poor blood pressure control.
During the trial, symptomatic hypoglycemia was not reported. Due to elevated triglyceride levels (594 mg/dl) in May 2021, fenofibrate was started. Lactate levels remained elevated (7.2 mmol/l), uric acid was 8.3 mg/dl and the plasma proteinogram remained unchanged.
The treatment resulted in reduction of the number of medications prescribed for concomitant clinical conditions. In 2017, 2018 and 2019 the patient required 9, 10 and 11 drugs, respectively. In 2020, before the empagliflozin administration the patient received 13 drugs. After empagliflozin initiation the number of drugs was reduced to 11 (excluding empagliflozin) and the G-CSF dose could be reduced.
Patient No. 2. Course of the disease before treatment with empagliflozin
The younger brother was diagnosed with GSD Ib at birth. During childhood, he developed HLA-B27-positive arthritis with axial and peripheral joint involvement. Treatment with oral glucocorticoids and methotrexate was started; however, arthritis flare-ups lasting 2–3 weeks/flare-up still occurred. Because of bilateral patellar dislocation, he has been wheel-chair-bound since 2017. He was also diagnosed with severe osteoporosis and zoledronic acid was started.
During childhood, he underwent repeated esophagus dilatation due to recurrent strictures. He was diagnosed with IBD at 6 years of age and treated with mesalazine that was replaced with sulfasalazine when arthritis developed. The patient had about 3 normal stools per day, and seldom complained of abdominal pains. Abdominal MRI revealed enlarged liver and spleen.
Since 10 years old, the patient has been receiving G-CSF due to neutropenia and recurrent infections. From the age of 14 years the patient required G-CSF on a regular basis, mean 0.3 mg/day. Despite the treatment with G-CSF, in 2017 the white cell count was 1.96 K/µl with ANC 0.27 K/µl. Normocytic anemia also developed. He was diagnosed with multiple myeloma in February 2020. No signs or symptoms of extramedullary involvement were detected, and the hematologist did not recommend a specific therapy. In July 2020, before empagliflozin treatment, the white cell count was 2.87 K/µl with ANC 0.91 K/µl. Hemoglobin concentration was 9.4 g/dl and mean cell volume 90.4 fl.
There were no symptoms of heart failure. Due to sinus tachycardia, metoprolol and ivabradine were administered to control the heart rate. The echocardiographs revealed normal findings. NT-proBNP concentration was 350 pg/ml.
Nephromegaly (L = 13.6 cm, R = 12.2 cm), glomerular hyperfiltration, and microalbuminuria (albumin to creatine ratio 44 mg/g) were detected in 2017.
While on the cornstarch diet, hypoglycemia occurred several times per year, and levels of lactate (6.5 mmol/l) and triglycerides (242 mg/dl) remained elevated. Hyperuricemia (uric acid 7 mg/dl) was treated with allopurinol. In July 2020, before empagliflozin administration, his triglycerides were 92 mg/dl, uric acid 5.1 mg/dl.
Course of the disease after empagliflozin administration
Empagliflozin was started when he was 28 years old. By that time, his body weight and BMI were 44 kg and 19.6 kg/m2, respectively. During the trial, the patient gained 3.5 kg due to improved appetite.
The neutrophil count increased during the first weeks and allowed for dose reduction of G-CSF to 0.3 mg twice a week. Hemoglobin level increased, and CRP normalized (Figure 1).
During 12 months of empagliflozin treatment he denied episodes of abdominal pain. The stool frequency and consistency remained unchanged. Magnetic resonance showed hepatosplenomegaly with no changes in diameters of these organs. No upper respiratory tract infection occurred. He was vaccinated with the BNT162b2 mRNA COVID vaccine without any adverse events.
Within the first week of therapy, pain and edema of joints resolved. Glucocorticoid dosage was gradually tapered from 4 mg of methylprednisolone and discontinued on the 137th day of research. Sulfasalazine dose was reduced from 3 g/day to 2 g/day.
During empagliflozin treatment there were no signs or symptoms of heart failure. Ivabradine was discontinued. De-escalation in heart failure treatment has not resulted in a recurrence of resting tachycardia. NT-proBNP concentration decreased to 82 pg/ml.
During the trial, symptomatic hypoglycemia was not reported. Triglycerides were 202 mg/dl, uric acid 4.7 mg/dl. The lactate level remained unchanged.
After 12 months of empagliflozin treatment, conjunctivitis, keratitis, and iritis developed, which were treated with local glucocorticosteroids and antibiotics. The G-CSF dose was also temporarily increased (up to a dose of 0.3 mg/day). Afterwards the G-CSF dose was reduced to 0.3 mg twice a week.
The number of drugs prescribed for associated clinical conditions was reduced. In 2017, 2018 and 2019 the patient required 13, 14 and 12 drugs, respectively. In 2020, before the treatment experiment the patient received 12 drugs. After empagliflozin initiation the number of drugs was reduced to 9 (excluding empagliflozin) and the G-CSF dose could be lowered.
Discussion
We confirmed the effectiveness of empagliflozin treatment in immunodeficient GSD Ib patients as it improved signs and symptoms of IBD, arthritis and anemia. Also the concentration of CRP, an inflammation marker, normalized during empagliflozin treatment. Although the improvement of neutrophil function was associated with enhanced 1,5AG6P elimination, the mechanism of reduced inflammation remains unknown.
We have presented two adult GSD Ib patients with multiorgan involvement. There are few case reports regarding empagliflozin treatment of GSD Ib, mostly involving children, that resulted in G-CSF discontinuation or dose reduction, anemia resolution, reduced spleen size, and IBD symptoms, healing of chronic wounds, and lower fecal calprotectin excretion [8–11].
Cardiovascular system involvement is not frequently reported in GSD Ib but was present in our patients. During the empagliflozin treatment the standard pharmacotherapy of heart failure or tachycardia was tapered without worsening of signs and symptoms. The mechanisms behind these effects remain unclear and may be related to increased hemoglobin levels or postulated cardiovascular benefits of flozins [12].
In diabetic and non-diabetic subjects SLGT2 inhibitors reduce plasma triglyceride levels and slightly increase low-density lipoprotein and high-density lipoprotein cholesterol. In our patients, the administration of empagliflozin was associated with marked hypertriglyceridemia, greater in the brother who lost weight during the study. Hypertriglyceridemia is observed in obese subjects with high visceral adipose fat [13] and may be related to lipolysis and release of glycerol and fatty acids to compensate for glycosuria induced by the SLGT2 inhibitor. This mechanism may be exacerbated in GSD Ib due to intracellular glucose deficiency caused by an absence of glucose-6-phosphate transporter.
Although the optimal empagliflozin dose to treat GSD Ib has not been established, 10 mg is routinely used in diabetic patients [14]. Since evidence regarding empagliflozin in GSD Ib is mostly limited to pediatric patients, the dose is usually calculated per kilogram of body weight. The patients with GSD Ib have been treated with the maximum dose of 20 mg/day; based on the patients’ body weight, the minimum and maximum doses are 0.3 mg/kg and 0.7 mg/kg, respectively [8]. In patients 1 and 2, the calculated doses were 0.28 mg/kg and 0.46 mg/kg, respectively, which corresponded to the regimen described by other studies; however, the dose for patient 1 was relatively low. Conversely, patient 1 was the heaviest among the reported GSD Ib patients treated with empagliflozin. Future studies should assess whether the empagliflozin dose should be based on the 1,5AG6P plasma level [7].
Over a 12-month follow-up period, empagliflozin use in GSD Ib patients was shown to be safe. Before therapy, the patients’ metabolic control and general health were favorable, and the incidence of hypoglycemia was low. Thus, the treatment prior to initiating empagliflozin could be considered as optimal. Nevertheless, empagliflozin improved the patients’ well-being, allowed for dose reduction of G-CSF, and reduced the symptoms and number of concomitant medications.
The main limitation of our report is the lack of clinical trial design. However, despite the lack of adequate controls, our data confirm those of previous reports regarding the clinical benefits of empagliflozin in patients with GSD Ib. We also did not measure levels of 1,5AG6P or neutrophil oxygen burst. The optimal dose of empagliflozin and the use of other potential SGLT-2 inhibitors have not been established [15]. These issues may be resolved by the results of ongoing clinical trials, such as a large study (NCT04930627) conducted in Poland by the Children’s Memorial Health Institute in collaboration with the Medical University of Warsaw.
In conclusion, empagliflozin treatment in subjects with GSD Ib is safe, associated with reduction of symptoms and allows the G-CSF dose and number of concomitant medications to be reduced.