Introduction
The neuropeptide oxytocin (OT) has been recognised not only for its hormonal activity in the regulation of reproduction, but also for its anxiolytic properties and beneficial effects in social behaviour and autism [1–3]. Recent human studies have provided evidence for a possible correlation between intravenous administration of oxytocin during labour and increased oxidative stress [4]. Furthermore, it has been reported that increased oxidative stress during preterm labour can be reduced by administration of the oxytocin receptor (OTR) antagonist atosiban [5].
In the last few years, special attention has been focused on oxytocin as a cardiovascular hormone [6]. The presence of OT and its receptor was demonstrated in brain regions such as the solitary tract nucleus (NTS) and rostral ventrolateral medulla (RVLM) essential for regulation of the cardiovascular functions [7, 8]. Centrally administered oxytocin effectively buffered the pressor reaction to acute stress in rats with myocardial infarction and arterial hypertension [8–10]. Gutkowska et al. reported sustained diurnal and nocturnal hypotensive effects of intravenously infused Oxytocin in spontaneously hypertensive rats [11]. Accumulating evidence points to the presence of OT and its receptor within the cardiovascular system, specifically in the heart and great vessels [12, 13]. OT in the heart has been shown to exert cardioprotective effects in experimental models of cardiac injury, and its activity increased in the cardiac muscle of rats developing post-myocardial infarction heart failure [14–16]. Recent studies have emphasised the anti-hypertrophic effect of OT. In particular, it has been shown that OT and its precursor OT-Gly-Lys-Arg (OT-GKR) abolish the development of cardiac muscle hypertrophy via cGMP/protein kinase G pathway in ex vivo cultures of the cardiomyocytes [17]. Therefore, the spectrum of cardioprotective actions of OT broadens in the context of attenuation of cardiac hypertrophy, especially due to arterial hypertension. Unfortunately, to date, the expression of the oxytocin system in the heart with left ventricle hypertrophy in arterial hypertension has not been evaluated.
The present study aimed to determine whether the presence of cardiac hypertrophy due to arterial hypertension is associated with a change of activity of the oxytocinergic system in cardiomyocytes.
Material and methods
Animals
Experiments were performed on 22 rats weighing between 270 and 335 g: 12 male, spontaneously hypertensive rats (SHR) and 12 normotensive Wistar-Kyoto rats (WKY). All rats were obtained from the Department of Animal Breeding at the Medical University of Warsaw. Prior to the experiments the animals were housed (2–3 animals per cage) under standard conditions including 12 h/12 h light/dark cycle (light on at 7.00 am) in a room with regulated temperature (range: 22–25°C) with access to a standard rat pellet diet and tap water ad libitum. All surgical and experimental protocols were conducted according to the domestic and the European Union guidelines and regulations on the use and care of laboratory animals (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010).
Selection of the animals
Before the tissue harvesting, non-invasive blood pressure measurement with tail-cuff method (CODA, Kent Scientific Corp., Torrington, USA) was performed to confirm the presence of arterial hypertension in SHR rats and normotension in WKY rats. The blood pressure measurements started after a 10-minute rest period; 10 measurements were taken from each animal.
Tissue harvesting and post-mortem examination procedures
All the animals were sacrificed by decapitation under general anaesthesia (100 mg/kg body wt. ketamine with 1 mg/kg body wt. xylazine, intraperitoneally, Vetoquinol Biowet). At least 2 ml of blood was collected from each rat before heart excision. Blood was centrifuged (5000 rpm) at 4°C for 10 min. The supernatant (plasma) was then decanted and stored at –80°C. The heart was excised from the thorax, ventricles were separated from the atria, and the left (LV) and right (RV) ventricle were separated along the longitudinal axis. Both ventricles were weighed, and all tissue fragments were placed immediately in liquid nitrogen. Frozen tissue fragments and plasma were kept in a deep freeze at –80°C.
Immunocytochemistry and image acquisition
Heart cryostat sections (20 μm thick) were cut at –20°C (Leica CM1850, Leica, Germany) and mounted directly on slides, then dried and fixed with 4% PFA (30 min), permeabilised with 0.25% Triton X-100, and blocked with 10% donkey serum +1% BSA. The following primary antibodies were applied overnight: OTR (Abcam, ab87312, 1 : 200), a skeletal muscle actin (a-SMA) (Abcam, ab28052, 1 : 100). After washing with PBS, appropriate secondary antibodies (Donkey anti-Mouse IgM (H + L) Alexa Fluor® 594 or Donkey anti-Goat IgG (H + L) Alexa Fluor® 488, 1 : 500, ThermoFisher Scientific) were applied for 2 h at room temperature (RT). Cell nuclei were contra-stained (20 min/RT) with Hoechst 33258. Images were obtained using a Zeiss Confocal Laser Microscope LSM 710 (Zeiss LSM 710, Carl Zeiss, Oberkochen, Germany) with a coherent multiphoton unit.
OT and OTR mRNA expression (real-time PCR)
The fragments of left and right ventricle were homogenised in TRIzol® Reagent (Ambion, Life Technologies) at a frequency of 25 Hz for 5 min in a TissueLyser LT (Qiagen GmbH, Hilden, Germany) homogeniser. Isolation of total RNA was performed using PureLink RNA Mini Kit (Ambion, Life Technologies), according to the manufacturer’s instructions. RNA concentration and purity were assessed with a spectrophotometer at 260 nm (SmartSpec Plus, Bio-Rad). Real-time PCR was performed on a ViiA™ 7 Real-Time PCR System using TaqMan® RNA-to-CT™ 1-Step Kit (Applied Biosystems, Foster City, USA). Specific primer and probe sets were purchased from Applied Biosystems: oxytocin (gene symbol Oxt, accession no. Rn00564446_g1; forward 5’-GACGGTGGATCTCGGACTGAA-3’, reverse 3’-CGCCCCTAAAGGTATCATCACAAA–5’); oxytocin receptor (gene symbol Oxtr, accession no. Rn00563503_m1; forward 5-GTCAATGCGCCCAAGGAAG-3’, reverse 3’-GATGCAAACCAATAGACACC-5’) labelled with FAM reporter dye. Glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems gene symbol Gapdh, accession no. Rn01775763_g1; forward 5′-AATGGTGAAGGTCGGTGTGAAC-3′ reverse 5′-AGGTCAATGAAGGGGTCGTTG-3′) labelled with VIC was used as a housekeeping gene. The experiment was conducted using MicroAmp™ Optical 96-Well Reaction Plates with Barcode (Applied Biosystems). The total reaction mixture (50 μl) was subjected to amplification under the following conditions: 40 cycles at 95°C for 15 s and at 60°C for 1 min. Duplicates of each sample were performed. The Ct (threshold cycle) for the target gene and the Ct for the internal control were determined for each sample. The relative gene expression was derived from the estimations of the values of the delta cycle threshold (ΔCt) by relative quantification to the endogenous control.
The protein level of OT and OTR (ELISA)
For the measurements of the OT and OTR protein concentration in the heart’s homogenates following EIA Kits were used: Oxytocin EIA Kit (Phoenix Pharmaceuticals Inc., USA), Oxytocin Receptor EIA Kit (Sunred Biological Technology Co. Ltd., China). Each sample of tissue was homogenised in a TissueLyser bead mixer (Qiagen, USA) and centrifuged (10,000 rpm, 10 min, 4°C). The supernatant was collected and frozen at –80°C until the analysis. Total protein concentration was measured using bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Holland), according to the manufacturer’s instructions. Results obtained were presented as an absolute ratio: concentration/total protein concentration (×10–9) for OT, OTR. Plasma OT concentration was measured for plasma samples from each group using an Oxytocin ELISA Kit (Phoenix Pharmaceuticals Inc., USA).
Statistical analysis
Statistica software version 12 (StatSoft Inc., Tulsa, OK, USA) was used for the analysis of the data. The distribution was checked for normality using the Shapiro-Wilk W test and homogeneity of variance using Levene’s test (ANOVA). The parameters with normal distribution were analysed with parametric tests (one-way ANOVA single and multivariate normal distributions). For parameters with non-normal distribution ANOVA Kruskal-Wallis, multiple comparison average ranks, and Mann-Whitney U test for independent samples were applied. The results/values presented in the text and figures are means ± standard errors (SE). The differences were considered statistically significant if p < 0.05.
Results
Body mass did not differ between SHR and WKY rats (280 g vs. 289 g; NS). The mean value of blood pressure in SHR group and in WKY group was 150/98 mm Hg and 105/76 mm Hg, respectively (F[1,21] = 37.287; p < 0.001). The left ventricle mass was higher in the SHR rats (0.85 ±0.02 g vs. 0.72 ±0. 017 g; F[1,21] = 13.92; p < 0.001), whereas no significant differences were found in the mass of the right ventricle between SHR and WKY rats (0.19 ±0.005 g vs. 0.2 ±0.004 g; NS). Plasma oxytocin concentration also did not differ between SHR (88.7 ±2.9 ng/ml) and WKY (89 ±3 ng/ ml) rats.
The OT mRNA expression was significantly higher in the left (F[1,10] = 13.155; p < 0.01) and in the right ventricle (F[1,12] = 21.454; p < 0.01) in the rats with arterial hypertension (SHR) (Figure 1 A) and was accompanied by higher levels of OT protein in the cardiac muscle of the left (F[1,12] = 157.35; p < 0.001) and right (F[1,12] = 9.314; p < 0.01) ventricle in SHR rats (Figure 1 B).
Figure 1
Average expression of oxytocin (OT) mRNA (A) and protein (B) in the left and right ventricle muscles in the in the normotensive Wistar-Kyoto (WKY) and in spontaneously hypertensive (SHR) rats. Relative expression: relative gene expression is given on the basis of estimations of the values of the delta cycle threshold (ΔCt) by relative quantification to the endogenous control. Oxytocin concentration presented as an absolute ratio: oxytocin/total protein concentration
Means ± standard errors are shown. Significant differences between the experimental groups: **p < 0.01, ***p < 0.001.
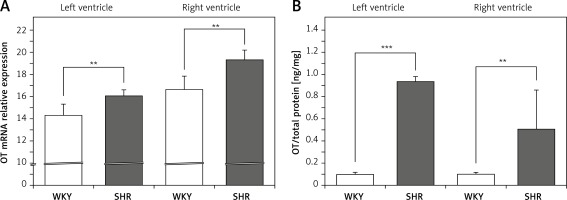
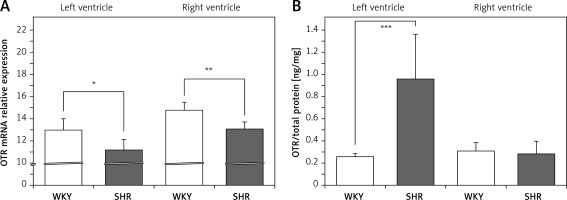
On the other hand, the expression of mRNA for OTR was significantly lower in the left (F[1,11] = 8.579; p < 0.05) and right (F[1,12] = 17.255; p < 0.01) ventricle in the SHR rats (Figure 2 A). However, OTR protein level was higher in the left ventricle in SHR rats (F[1,12] = 20.464; p < 0.001) (Figure 2 B). No differences in OTR protein level between SHR and WKY rats were found in the muscle of the right ventricle (Figure 2 B).
Figure 2
Average expression of oxytocin receptor (OTR) mRNA (A) and protein (B) in the left and right ventricle muscles in in the in the normotensive Wistar-Kyoto (WKY) and in spontaneously hypertensive (SHR) rats. Relative expression: relative gene expression is given on the basis of estimations of the values of the delta cycle threshold (ΔCt) by relative quantification to the endogenous control. Oxytocin receptor concentration presented as an absolute ratio: oxytocin/total protein concentration
Means ± standard errors are shown. Significant differences between the experimental groups: *p < 0.05; **p < 0.01; ***p < 0.001.
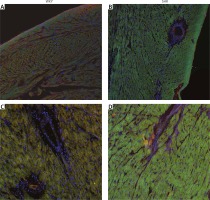
Immunostaining of the cardiac muscle for OTR confirmed the results obtained with ELISA and showed higher expression of OTR in the muscle of the left ventricle in SHR rats (Figure 3). Moreover, the tissue samples of the left ventricle in SHR rats revealed hypertrophy of the cardiac muscle cells, which also corresponds with higher left ventricle mass in SHR rats (Figure 3).
Figure 3
Immunostaining of the cardiac muscle of the left ventricle in the normotensive Wistar-Kyoto (WKY; A, C) and in spontaneously hypertensive (SHR) rats (B, D). Figure shows hypertrophy of the left ventricle cardiomyocytes and high intensity of oxytocin receptor (OTR; green staining) in the myocytes of the left ventricle in the SHR rats. Staining of the cell nuclei with Hoechst 33258 (blue), oxytocin receptor OTR (green), and α-skeletal muscle actin – aSMA (red). The bar – 100 μm
Discussion
The present study demonstrates for the first time an increased activity of the oxytocinergic system in the cardiac muscle and, specifically, higher levels of oxytocin and its receptor in the left ventricle in conjunction with hypertension-related hypertrophy of the cardiac muscle in SHR rats.
Our results thus suggest a relationship between arterial hypertension and left ventricle hypertrophy and are in agreement with both animal and clinical data, showing that the presence of arterial hypertension contributes to cardiac remodelling and left ventricle hypertrophy and is a potent and indisputable risk factor for the development of chronic heart failure, with preserved ejection fraction in particular [17, 18]. The hypertrophic response of the myocardium in arterial hypertension is a complex response to biomechanical stress (stretching) and biological factors such as angiotensin II, enhanced endothelin-1 release, vasopressin, neuropeptide Y, inflammatory cytokines, and norepinephrine [19, 20]. Present studies suggest an additional correlation between high levels of androgens and diastolic dysfunction of the left ventricle [21].
Recently, attention has been focused on oxytocin as a cardioprotective and cardiomyogenic factor with antihypertrophic potential [16, 22]. In our study, OT mRNA expression and OT protein level were significantly elevated in both left and right ventricle in rats with arterial hypertension. Moreover, we have also demonstrated a high level of OTR protein in the muscle of the left ventricle, suggesting that increased OT level can act directly via increased OTR receptors. This leads us to the conclusion that the activity of the oxytocinergic system is increased in the left ventricle of hypertensive rats. To date, the expression of the OT in the heart was described only in normotensive rats [12, 23, 24]. However, in our previous study, we demonstrated increased expression of OT in the hearts of rats developing post-myocardial infarction heart failure [24], where this effect was potentially associated with the antifibrogenic, antihypertrophic, anti-inflammatory effect resulting in the reduction of myocardial damage [14, 15, 22]. In the study by Menaouar et al. oxytocin and its precursor OT-Gly-Lys-Arg (OT-GKR) blocked endothelin-1-induced cardiac hypertrophy ex vivo by activating cGMP/protein kinase G pathway and the PI3K/Akt pathway, considered essential for physiological growth but also for inhibition of pathological hypertrophy in cardiomyocytes [16, 25]. Garrott et al., using a rat model of left ventricle hypertrophy induced with trans-ascending aortic constriction, reported that activation of hypothalamic OT neurons improved cardiac contractility and reduced cellular hypertrophy, interleukin-1b levels, and fibrosis in the myocardium [26]. Our data suggest that an increase in OT in the ventricles of hypertensive rats may result from an ongoing process of cardiac remodelling and cardiomyocyte hypertrophy. However, as there are still few data published regarding the expression and role of the oxytocinergic system in the pathogenesis and progression of cardiac hypertrophy/remodelling in arterial hypertension, so further investigation in this area is essential.
Surprisingly, high expression of the OT was not limited to the left ventricle; we also observed higher protein level and OT mRNA expression and protein level in the muscle of the right ventricle. Both clinical and experimental studies provide evidence that the process of right ventricular remodelling develops in parallel with a similar process occurring at the left side, but the remodelling progress is slower [27, 28]. In a study with magnetic resonance heart imaging, Todiere et al. showed that systemic arterial hypertension was associated with impaired diastolic function and concentric remodelling of the right ventricle [28]. Changes in right ventricular geometry and function deterioration were also described in the echocardiographic study in the population in patients with arterial hypertension. The authors of this study observed that the greatest deformation of the right ventricle is observed in cases of eccentric or concentric left ventricle hypertrophy [29]. In the present study, we did not find significant differences in the right ventricular muscle mass between SHR and WKY rats. Also, no differences were found in the level of OTR protein between hypertensive and normotensive rats. This may also be related to the lack of right ventricle hypertrophy observed in our study.
Our data showing decreased OTR mRNA and increased OTR protein level in the muscle of the left ventricle indicate a dissociation between OT mRNA and OTR protein level in rats with arterial hypertension, as described previously [24]. Possible mechanisms of the observed phenomenon may be related to desensitisation and internalisation of the OTR [30]. Other mechanisms may be related to suppression and destabilisation of mRNA involving the regulator RNA-binding proteins or microRNAs [31–33].
Our study has some limitations due to the relatively small sample size, and a follow-up study with a larger sample size would improve the statistical significance of our results. Consequently, further studies of anti-hypertrophic functions of oxytocin and the changes of the oxytocinergic system in the heart, using more direct methods, are warranted to clarify the critical role of oxytocin in maintaining cardiovascular homeostasis and in the pathophysiology of hypertension-related cardiac remodelling. The main finding of the present study is the association between arterial hypertension an increased activity of the intracardiac oxytocinergic system. This finding supports the data showing that oxytocin exerts pleiotropic effects, including both cardiomyogenic and antihypertrophic effects [16, 34] in the cardiovascular system, and plays an important role in maintaining cardiovascular homeostasis.
In conclusion, our data suggest that the increased activity of the oxytocinergic system in cardiomyocytes, especially in the left ventricle, may inhibit or at least slow down the progression of cardiac remodelling in arterial hypertension.
The epidemiology of the arterial hypertension is alarming. In 2015 arterial blood pressure was the leading global cause of premature deaths, accounting for about 10 million deaths [35]. Multifaceted modifications of lifestyle to prevent development of arterial hypertension and subsequently to decrease the rate of hypertension-related complications are necessary [36]. Hypertension-mediated heart damage such as left ventricle hypertrophy and remodelling can worsen the prognosis of patients with arterial hypertension. Because oxytocin plays both cardiomyogenic and antihypertrophic roles, it may have potential for therapeutic application in hypertension-related heart failure and contribute to the reduction of mortality and comorbidity due to arterial hypertension.