Two-dose mRNA SARS-CoV-2 vaccines are unable to elicit a sufficient immune response in immunocompromised individuals. In kidney transplant recipients (KTRs), we previously demonstrated a markedly impaired seroconversion rate of 51.4% for anti-spike IgG antibodies after two doses of basic mRNA vaccination. The independent predictors of no response were older age, shorter transplantation vintage, and stronger immunosuppressive protocols. In addition, the magnitude of the anti-body-mediated response in seroconverted individuals was much lower than that in the immunocompetent controls [1]. This is very unfavorable in terms of prognosis, taking into account the very high susceptibility to SARS-CoV-2 infection, severe course of the disease and the high mortality rate in KTRs. The threat may increase as new virus variants emerge with increased transmissibility and a more severe disease course [2]. Therefore, many national health authorities recommend the third – complementary – dose as a regular course of vaccination with an mRNA platform for immunocompromised individuals. Although seroconversion may be achieved after the third dose by almost half of the patients in whom the primary vaccination failed, still nearly one third of patients remain seronegative and thus inadequately protected against COVID-19 [3]. The chance to achieve seroconversion is given by using successive boosters, namely the fourth or even fifth doses of the same vaccine [4, 5]. Increasing the dose of antigen in the vaccine may also be more immunogenic in a solid organ transplant recipient as suggested by influenza vaccination observations using inactivated virus type vaccines [6].
Our previous studies have shown that a better immune response during primary and complementary vaccination against COVID-19 can be achieved using formulations with higher mRNA doses, e.g. mRNA-1273 containing more than three times the mRNA than BNT162b2. Each dose of BNT162b2 delivers only 30 μg of mRNA, while each dose of the mRNA-1273 vaccine contains 100 μg [1, 3].
Methods
Therefore, we undertook a retrospective evaluation of the efficacy of a heterologous mRNA booster with mRNA-1273 in individuals who were constantly seronegative after three doses of the BNT162b2 vaccine. We screened 37 KTRs vaccinated with two doses of BNT162b2 (Comirnaty, Pfizer/BioNTech) 1 month apart who received a single, third – complementary – dose of the same vaccine 3 months after the second one according to the health authorities recommendations. Five months after the third dose the seronegative patients were offered the fourth vaccination with an mRNA-1273 (Moderna) booster. Quantitative determination of specific IgG antibodies to trimeric S-proteins as an indicator of the humoral response to vaccination was assessed 14-16 days after consecutive vaccine doses with a commercial chemiluminescent immunoassay kit (The LIAISON SARS-CoV-2 TrimericS IgG test, DiaSorin, Italy) as previously described [3]. Samples were interpreted as positive (seroconversion) or negative (no seroconversion) with a cutoff index value of > 33.8 binding antibody units (BAU)/ml according to the manufacturer.
Statistical analysis
Continuous data are expressed as mean and standard deviation. Categorical variables are presented as counts (percentages). Continuous variables were first tested for normal distribution using the Shapiro-Wilk test, and then compared by the t-test if normally distributed, and by the Mann-Whitney or Wilcoxon tests, where appropriate, if non-normally distributed. The χ2 test was used for categorical variables. The data were analyzed with Statistica (version 12.0, Stat Soft, Inc., Dell Software, Tulsa, OK, USA). P-values of < 0.05 were considered statistically significant.
Results
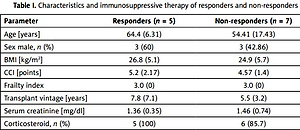
After the third dose of BNT162b2, 16 out of 37 patients (43.24%) had become seropositive with BAU > 33.8 BAU/ml. Finally, 12 constantly seronegative KTRs with no prior SARS-CoV-2 infection who had agreed to further vaccination received an mRNA-1273 booster 5 months after the third dose of BNT162b2. Their mean age was 58 years, mean time from transplant was 6.46 years, mean Charlson comorbidity index was 4.83, mean serum creatinine level was 1.42 mg/dl, and 50% were men. Their three-drug maintenance therapy consisted of a calcineurin inhibitor, antiproliferative drug, and a steroid. Detailed characteristics of the patients are presented in Table I.
Table I
Characteristics and immunosuppressive therapy of responders and non-responders
A total of 5 out of 12 patients (41.7%) seroconverted after the mRNA-1273 booster (fourth dose), with a mean BAU titer of 353/ml assessed 14–16 days after vaccination. Of note, 3 of them had a high BAU titer (> 264/ml), which can be considered as neutralizing [7]. Neither serious adverse events nor graft rejections were observed within 2 months after the booster. Serious adverse events were defined as any untoward medical occurrence that resulted in death, was life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, or resulted in persistent disability/incapacity [8].
Discussion
The study demonstrated that the administration of a heterologous booster with the mRNA-1273 vaccine in KTRs who were constantly seronegative after three doses of the BNT162b2 mRNA vaccine may show immunogenic potential. Seroconversion was achieved in almost half of the patients in whom the primary three-dose vaccination failed. The results are consistent with several previous reports showing an increase of the seroconversion rate in KTRs after the fourth or fifth dose of a homologous mRNA booster [4, 5]. Given the observational nature of our study, we were not able to assess whether the positive immunogenic effect observed in some of our patients (seroconversion) resulted from the next dose of the vaccine or the use of the different preparation with a higher mRNA content. This should be the subject of further controlled studies. Another issue to consider is the possibility of delayed antibody production after vaccination in immunocompromised individuals [9]. Hence, in the absence of seroconversion in KTRs, an additional evaluation of humoral response should be considered later, after vaccination. Our study also has a number of limitations, including the small sample size and the observational design that limits the conclusions that can be drawn about causality.
Regardless of these issues, our study showed that the administration of a heterologous mRNA vaccine formulation with a high mRNA dose as a consecutive booster may be an effective alternative for achieving post-vaccination immunity in KTRs who remain seronegative after the primary vaccination regimen. It seems, however, that in some KTRs it will be impossible to obtain active immunization at all or it will be at low levels. In such cases, mandatory use of barrier measures by these patients is a must, and administration of monoclonal anti-s antibodies may be considered as a pre-exposure or post-exposure prophylaxis to prevent COVID-19 infection [10–12].