Introduction
Titanium dioxide TiO2 is a substance used extensively nowadays in various fields, for example as an antibacterial agent [1]. It is still being studied regarding its usage and potential use in the future. Its various effects on the environment are being monitored extensively to detect possible complications of its usage.
Nowadays TiO2 is being used in the food industry as a food additive. The effect of TiO2 on the gastrointestinal system as a food additive has been considered safe and shows no major effect in vitro on intestinal bacteria such as Bacteroides ovatus and Clostridium cocleatum, suggesting its safety for use in the intestine [2]. Besides that, the various and different forms of titanium dioxide (TiO2) may influence its action in the human body [3].
Interestingly, various studies have suggested a negative influence of TiO2. A study on Balb/c mice showed the effect of nano-titanium dioxide caused cytotoxicity and possible effect to the cardiovascular system of the mice, which is shown to be countered by vitamin E [4]. In addition, nano-titanium dioxide produced by sol-gel method showed an impact on earthworm Eudrilus euginiae proliferation and the survival possibly of reactive oxygen species (ROS) formation from TiO2 [5]. Furthermore, nutritional starvation and drought stress are 2 effects of TiO2 on the plant stress response [6]. Interestingly, an in vitro test using alveolar epithelial cells and macrophage showed decreased reactive oxygen species (ROS) by adding silica to the TiO2, which was suggested to modify the effect of the TiO2 [7].
A study of the effect of nanotitania on the aquatic ecosystem revealed a negative effect of the TiO2 on the nucleus and cell membrane together with organelle damage to the freshwater algae [8]. Specifically, the order Cladocera aquatic species is speculated to be prone to be affected by TiO2 phototoxicity [9].
This substance was developed to be used as an antimicrobial agent. Our substance has been tested against the growth of Staphylococcus aureus in the lab [10]. In addition, it has been shown to have no mutagenic effect by using Ames test for genotoxicity test [11]. In this study, our nanotitania extraction with 0.05% silver was tested against L929 mouse cells, aimed at testing its toxicology effects in in vitro experiments.
Material and methods
Material preparation
The nanotitania extraction with 0.05% silver was developed by Universiti Malaysia Sabah (UMS), as mentioned in a previous publication [12]. Our extraction is non-water based; it is dimethyl sulphoxide (DMSO) non-dilution. The extracted nanotitania have been experimented using high-resolution scanning electron microscopy (SEM), high-resolution transmission electron microscopy for the particle crystallinity quality, and X-ray diffractometer for nanotitania anatase confirmation.
Cytotoxicity evaluation of nanotitania extract
The cytotoxic effect of nanotitania extract was evaluated by MTT assay. The sample nanotitania extract with 0.05% silver was sterilised using autoclave at 121 ±1oC for 20 min. Mouse Fibroblast cell line (L929) was plated in 96-well plates at 1 × 104 cell density in completed growth medium and subject to incubating process at 37 ±1oC for 24 ±2 h. Then 100 μl extracted solution of material at 100, 50, 25, 12.5, 6.3, 3.1, and 1.5 mg/ml was replaced in the 96-well plates and incubated at 37 ±1oC for 24 ±2 h. Zinc sulphate was used (0.46, 0.23, 0.115, 0.058, 0.029, 0.014, 0.007 mg/ml) as a positive control material. After 24 h, 10 μl of the MTT solution was added to each well and was subjected to 4 h of incubation. Cell with media only were used as a negative control. Post-incubation (4 h), 100 μl solution was eliminated from each well and swapped with 100 μl of DMSO. The plate was shaken using a shaker at 300 rpm for 15 min, and the outcome was read out using an ELISA reader spectrophotometer at 570 nm wavelength.
The cell viability (CV) was calculated according to the following formula:
The negative control used in this test was a medium of cell culture known as Dulbecco’s Modified Eagle Medium (DMEM + 10% FCS), incubated for 24 h under the same conditions as the extract.
The positive control used in this test was a dilution series of zinc sulphate (0.46, 0.23, 0.115, 0.058, 0.029, 0.014, 0.007 mg/ml), incubated for 24 h under the same conditions as the extract.
Statistical analysis
Statistical assessment of the data was achieved by using one-way analysis of variance (ANOVA) using SPSS version 26.0 software to evaluate the significance of different concentrations of 0.05% nanotitania extract to L929 cell lines. The significant discrepancy between different concentrations of 0.05% nanotitania extraction for cytotoxicity valuation with p < 0.05 was considered significant. Non-linear regression analysis of Graphpad Prism software version 6 was used to determine the IC50 value.
Results
Cytotoxic effect and IC50 of 0.05% nanotitania on normal cell lines
The cytotoxic effects of 0.05% nanotitania was analysed on L929 cell lines with different concentrations for 24 h of incubation period. The cytotoxic effect of the samples was evaluated using MTT assay. The cell viability of cell lines used in this study was above 70% confluent before treatment. Our results show that most of the nanotitania dilutions (1.5, 3.1, 6.3, 12.5 and 25 mg/ml) were not significantly changed compared to negative control.
The 0.05% nanotitania at concentrations of 1.5, 3.1, 6.3, 12.5, and 25 mg/ml is explained as follows: after the nanotitania extraction was made, it was observed that at certain values of e.g. temperature, acid molarity, and mass ratio, the growth of nanotitania is at its optimum. In order to produce good, high crystallinity nanoparticles, a method of modified hydrothermal imposed with molten salt method was introduced during the extraction synthesis. For this molten salt process, a silver nitrate was used, added by 0.05% by weight to the extracted nanotitania.
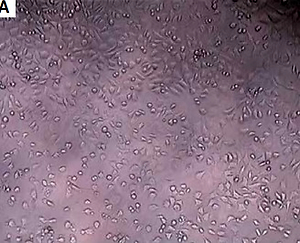
Only the concentrations of 50 and 100 mg/ml were significantly distinct compared to the negative control in Figure 1. This result revealed that there was non-cytotoxicity activity of 0.05% nanotitania at concentrations 1.5, 3.1, 6.3, 12.5, and 25 mg/ml on L929 cell lines, but not at concentrations 50 and 100 mg/ml. The result was related to the OD (optical density) reading, in which a higher OD reading indicated that a higher percentage of viable cells, and a lower OD reading indicated a lower percentage. Furthermore, the inhibitory effect of 0.05% nanotitania at different concentrations with an IC50 value of 3.215 mg/ml at 24 h (R2 = 0.9157) is shown in Figure 2.
Figure 1
Cytotoxicity effects of 0.05% nanotitania extract at concentration 1.5–100 mg/ml on L929 cells after 24-hour incubation. Each value represents mean ± SEM of 10 wells per concentrations in triplicates. A significant difference with negative control with p < 0.05 compared to negative control (NEG)
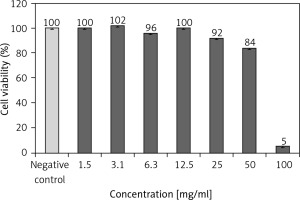
Figure 2
Determination of IC50 using MTT assay at 24 h. The curve was constructed by GraphPad Prism software version 6 using non-linear regression analysis to construct the curve of 0.05% nanotitania extract at different concentrations and the provided inhibitory effect on L929 cells with an IC50 value of 3.215 mg/ml at 24 h (R2 = 0.9157)
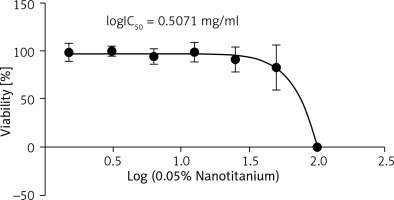
Evaluation of morphological changes of L929 cell line treatment with 0.05% nanotitania extract
Morphological changes of normal L929 cell line treated with 0.05% nanotitania extract at different concentrations (1.5–100 mg/ml) for 24 h were observed using an inverted microscope. Nanotitania extract with 0.05% silver had no cytotoxic effect in the growth inhibition test with L929 mouse except for the 100 mg/ml extract (Figure 3).
Figure 3
Morphological changes of the L929 cell after treatment with 0.05% nanotitania extract. A – negative control, B – positive control, C – 100 mg/ ml extract. Scalebar: 200 μm

The International Organisation for Standardisation (ISO 10993-5:2009) guideline was applied in the reporting of morphological changes.
Discussion
Hence, another test on cell viability and reactivity is done by using 0.05% silver in TiO2. The results of the material nanotitania with a combination of 0.05% silver in different concentrations is thought to show no cytotoxic effect in the growth inhibition test with L929 mouse besides the 100 mg/ml extract. Therefore, it means that TiO2 with a combination of 0.03% silver (which has a lower concentration) logically and inevitably has no cytotoxic effect and is safe to be used in future.
For the future benefit, it is best to suggest that this nanotitania be extracted via modified hydrothermal improve with molten salt process study under various virology tests to observe its disinfection ability. The limitation of this study was the ability to produce the substance on a mass scale, which is restricted by our small laboratory together with the very intense processes in producing it.
Apart from that, the non-cytotoxicity characteristic of the nanotitania is our main concern as well, to make sure itis safety for all clinicians and their patients in future. It also can be concluded that the process of producing the nano titanium dioxide a safe process overall.
In conclusion, nanotitania extract with 0.05% silver has no cytotoxic effect on the growth inhibition test with L929 mouse, except for the 100 mg/ml extract.